Hip osteoarthritis (OA) is a common and debilitating condition that imposes a significant health burden worldwide. The global incidence of hip OA has risen from 0.74 million in 1990 to 1.58 million in 2019.1 By 2060, the demand for hip and knee joint replacements is expected to increase by nearly 40%, with older patients comprising the largest demographic.2,3 Total hip arthroplasty (THA) is an effective solution for treating hip OA, offering relief from pain and improving mobility. However, to ensure successful outcomes, it is crucial to minimize complications such as infection, dislocation, and loosening while achieving high levels of patient satisfaction. The surgeon’s technical skills and judgment are paramount in conventional THA (COTHA). Surgeons use various instruments and techniques to prepare the bone, position the acetabular cup and femoral stem, and secure them. In contrast, robotic-assisted THA (RATHA) integrates computer systems and robotic arms to assist surgeons. The procedure began with detailed preoperative planning using advanced imaging techniques. The robotic system then assists with surgical precision by guiding the instruments according to the surgeon’s plan, thereby improving the accuracy of implant positioning. Several robotic-assist systems have been developed for orthopedic surgery, including ROBODOC, ROSA, MAKO, CASPAR, NAVIO, and Acrobat.4,5 The first robot-assisted hip replacement surgery was performed in 1992 using the ROBODOC system.
The potential advantages of robotic assistance in joint replacement surgery include a smaller incision size, which can lead to reduced pain, faster recovery, and reduced scarring. Imaging data are used to create a three-dimensional model of the pateint's anatomy, enabling surgeons to plan surgery more precisely and potentially acheive better outcomes.6 The robotic arm can provide greater precision and flexibility in implant positioning, which is crucial for optimal function and longevity. A higher proportion of RATHA cases achieved optimal acetabular cup inclination and anteversion angles, improving biomechanical stability.7 Robotic assistance led to a 30% reduction in leg length discrepancy compared with manual techniques, potentially lowering the risk of complications, such as gait abnormalities.8 The potential benefits of smaller incisions, faster recovery, and improved outcomes may contribute to higher patient satisfaction. Precise implant placement and reduced surgical trauma may contribute to longer-lasting implants. RATHA showed a slight reduction in early postoperative dislocation rates and comparable infection rates.9 The use of the MAKO robot in THA improves radiological outcomes by enhancing safe prosthesis placement. However, no significant differences were observed in terms of complications.10
Despite its potential benefits, there are some drawbacks to RATHA. Robotic systems and their associated technologies can be expensive, potentially increasing the procedure cost.11 Robotic surgery can sometimes take longer than conventional methods, particularly for surgeons who are new to the technology. Surgeons require specialized training to effectively use robotic systems, which can involve a significant learning curve. In addition, there is always the potential for technical malfunctions or limitations that could affect the procedure. Although short-term benefits are often observed, long-term data on the true advantages of RATHA are still being collected and analyzed. Based on current evidence, there is no significant difference in the clinical and functional outcomes between RATHA and COTHA.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.12 A comprehensive literature search was performed from PubMed, Scopus, and the Cochrane Library databases inception until 31 August 2024. The search strategy included the terms such as ῾total hip arthroplasty,’ ῾THA,’ ῾total hip replacement,’ ῾THR,’ ῾robotic assisted,’ ῾conventional,’ and ῾manual’. These terms were applied to all searchable fields (title, keywords, and abstract). The search strategy also incorporated Boolean combinations like ῾total hip arthroplasty’ OR ῾THA’ OR ῾total hip replacement’ OR ῾THR’ AND ῾robotic assisted’ AND ῾conventional’ OR ῾manual’. The search process was conducted independently by two reviewers, with a third reviewer consulted to resolve any discrepancies.
Studies were considered eligible if they met the following inclusion criteria: (i) randomized controlled trials (RCTs), retrospective studies, prospective studies, and cohort studies comparing RATHA with COTHA; (ii) studies involving patients > 18 years diagnosed with severe hip diseases such as OA, avascular osteonecrosis, rheumatoid arthritis, and Paget’s disease; (iii) patients who had undergone THA; and (iv) studies reported short- and long-term outcomes comparing RATHA with COTHA. Exclusion criteria were as follows: (i) non–English publications; (ii) case reports, case series, abstracts, review articles, systematic reviews, and meta-analyses; (iii) biomechanical or cadaveric studies; (iv) studies on revision THA; (v) studies involving patients with high-grade hip dysplasia; and (vi) studies with insufficient data to extract relevant information. The primary outcomes were clinical outcomes measured using patient-reported outcome measures (PROMs). The secondary outcomes included operative outcomes, complications, and radiological assessment.
This systematic review included a mix of study designs such as case-control studies, retrospective cohort analyses, and prospective trials, rather than limiting the inclusion to RCTs provides a broader evidence base. Non-RCTs, such as retrospective cohort studies and case-control studies, often provide valuable data, particularly in areas where RCTs are limited or infeasible due to ethical, logistical, or financial constraints. For instance, in RATHA, RCTs are relatively scarce because of the novelty of technology and the difficulty in randomizing patients to surgical techniques. Non-RCTs often reflect real-world clinical settings, capturing a wider spectrum of patient populations, surgeon expertise, and institutional variations. This inclusion enhances the external validity (generalizability) of the systematic review findings. Retrospective and prospective studies can provide larger sample sizes and longer follow-up periods, contributing valuable insights into outcomes such as long-term complications, revision rates, and learning curves for surgeons. When RCTs are unavailable or insufficient in number, non-RCTs provide preliminary evidence to guide practice and inform future high-quality research. However, non-RCT studies have some limitations including prone to selection bias, confounding variables, and retrospective reporting inaccuracies. The variability in study design, patient populations, and measured outcomes may complicate data synthesis and interpretation. Without randomization, non-RCTs may not establish causality as robustly as RCTs. To mitigate the limitations of non-RCT inclusion in this review, we conducted include rigorous quality assessment using validated tools such as the Cochrane Risk of Bias Tool, which assessed the quality of included studies and identify potential biases. 14 We also ensured transparent reporting, clearly stating that non-RCTs were included to provide a more comprehensive assessment due to the limited number of RCTs. Potential biases and limitations in the interpretation of findings were mentioned, considering the inclusion of non-RCTs. Finally, we focused on consistency by highlighting consistent trends or findings across study designs, as this reinforces the reliability of conclusions despite differences in study quality.
Two reviewers (the first and second authors) independently screened the titles, keywords, and abstracts of all studies identified in the search, using Covidence systematic review software to remove duplicates.13 The full texts of eligible studies were independently reviewed to confirm their suitability. Both reviewers independently extracted data from each study, focusing on patient demographics, study design, sample size, robotic system used, operating time, complications, PROMs, conflicts of interest, and funding sources. Any disagreements at this stage were resolved by a senior reviewer (the fourth author). To assess the quality of the included studies, we used the Cochrane Risk of Bias Tool. To assess the treatment effects of RATHA compared with COTHA, a variety of statistical methods were employed. Standardized mean differences (SMDs) with 95% CIs were calculated to quantify the magnitude and precision of the treatment effects. Forest plots were utilized to visually represent the SMDs and their corresponding CIs across multiple studies.
Results
We identified 364 citations from the three databases; of which 236 were removed as duplicates. The remaining 128 studies were reviewed based on the selection criteria. Only nine studies fulfilled all the selection criteria and were included in the qualitative analysis of this review. The screening process is detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart [Figure 1]. Table 1 summarizes the characteristics of all included trials. We included nine trials that were published from 2015 to 2024 and were conducted in Asia (n = 5),15–19 followed by the USA (n = 2),20,21 the UK (n = 1),22 and Italy (n = 1).23 The sample sizes of these trials ranged from 54 to 176 hips, and the follow-up time ranged from three months to 14 years. Two studies were secondary analyses of the same patient population at longer follow-up periods.16,20 The age of participants across the studies ranged from 32 to 85 years old. A total of 933 patients were assessed, 467 of whom underwent RATHA.
 Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart.
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart.
Table 1: Characteristics of included trials.
|
Lim 2015
|
South Korea
|
Randomized short-term outcome
study
|
N = 49
RATHA = 24
COTHA = 25
|
ROBODOC
|
24 months
|
Yes
|
|
Bargar 2018
|
USA
|
Randomized clinical trials
|
N = 67
RATHA = 45
COTHA = 22
|
ROBODOC
|
RATHA
13.8 years
COTHA
14.2 years
|
Yes
|
|
Nakamura 2018
|
Japan
|
Randomized clinical trials
|
N = 128
RATHA = 64
COTHA = 64
|
ROBODOC
|
11.25 years
|
No
|
|
Fontalis 2023
|
UK
|
Prospective cohort study
|
N = 100
RATHA = 50
COTHA = 50
|
MAKO
|
36 months
|
Yes
|
|
Tian 2023
|
China
|
Retrospective cohort study
|
N = 143
RATHA = 63
COTHA = 80
|
Seven-axis robot-assisted THA system
|
3 months
|
Yes
|
|
Xu 2024
|
China
|
Prospective randomized, multicentre, parallel-controlled
clinical trial
|
N = 111
RATHA = 56
COTHA = 55
|
LANCET
robotic system
|
3 months
|
Yes
|
|
Lu 2023
|
China
|
Prospective trial
|
N = 59
RATHA = 30
COTHA = 29
|
Single semiactive surgical robot (YUANHUA-THA)
|
3 months
|
Yes
|
|
Buchan 2024
|
USA
|
Retrospective cohort analysis
|
N = 176
RATHA = 85
COTHA = 91
|
ROSA
|
12 months
|
No
|
RATHA: robotic-assisted total hip arthroplasty; COTHA: conventional total hip arthroplasty.
The ROBODOC systems (Integrated Surgical Systems or Curexo Technology Corp., CA, USA) were used in three studies.15,16,20 The Mako Robotic-Arm assisted total hip™ (Stryker Corp, USA) was employed in two studies.22,23 One study utilized the ROSA® Total Hip System (Zimmer CAS, Montreal, Canada).21 The three Chinese studies used different robotic platforms, which included the Seven-Axis Robot-Assisted THA system (Jianjia, Hangzhou Jianjia Robot Co., Ltd.),18 the single semiactive surgical robot (YUANHUA-THA),17 and the LANCET Robotic system (Hangzhou Lancet Robo Co. Ltd).19 Table 2 illustrates the outcome level for risk of bias for the included trials.
Table 2: Risk of bias of included trials (outcome level).
|
Lim 2015
|
Unknown
|
Unknown
|
Unknown
|
Low risk
|
Low risk
|
Unknown
|
Unknown
|
|
Bargar 2018
|
Low risk
|
Unknown
|
Unknown
|
Unknown
|
High risk
|
Low risk
|
Unknown
|
|
Nakamura 2018
|
Low risk
|
Low risk
|
High risk
|
Unknown
|
Low risk
|
Low risk
|
Unknown
|
|
Fontalis 2023
|
High risk
|
Not applicable
|
Unknown
|
Unknown
|
Low risk
|
Low risk
|
Unknown
|
|
Buchan 2024
|
Not applicable
|
Not applicable
|
Unknown
|
Unknown
|
Low risk
|
Unknown
|
Unknown
|
|
Tian 2023
|
Low risk
|
Low risk
|
Low risk
|
High risk
|
Low risk
|
Low risk
|
Low risk
|
|
Lu 2023
|
Low risk
|
Low risk
|
Unclear risk
|
Unclear risk
|
Low risk
|
Low risk
|
Low risk
|
|
Xu 2024
|
Low risk
|
Unclear risk
|
Unclear risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
The primary outcomes were clinical outcomes measured using PROMs. Table 3 summarizes the PROMs evaluated in this systematic review, including the Harris Hip Score (HSS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score and the University of California-Los Angeles (UCLA) score. The HSS was evaluated in four studies,17–19,23 the WOMAC score was evaluated in three studies,15,17,20 and the UCLA in three studies.20–22 In summary, all comparisons in the forest plot for PROMs, including the HSS, the WOMAC, and UCLA scores [Figure 2a, b, and c], show CIs crossing zero, indicating no statistically significant difference between the RATHA and COTHA groups, either preoperatively or at the end of treatment.
Table 3: Patient-reported outcome measures of included trials.
|
HSS
Preoperative
RATHA
COTHA
p-value
SMD
95% CI
End of treatment
RATHA
COTHA
p-value
SMD
95% CI
|
Mean (range)
52 (37–61)
55 (41–60)
0.155
Mean (range)
93 (85–100)
95 (89–100)
0.512
|
|
|
Mean ± SD
53.55 ± 13.93
54.71 ± 9.52
0.718
-0.10
-0.61–0.41
Mean ± SD
96.81 ± 5.15
97.23 ± 4.26
0.740
-0.09
-0.60–0.42
|
Mean ± SD
53.95 ±14.47
51.43 ± 13.55
0.359
0.18
-0.15–0.51
Mean ± SD
89.03 ±7.72
88.76 ± 5.79
0.818
0.04
-0.29–0.37
|
Mean ± SD
59.31 ± 19.24
58.84 ± 20.23
0.8993
0.02
-0.35–0.40
Mean ± SD
87.92 ± 10.88
87.99 ± 11.19
0.9786
-0.01
-0.38–0.37
|
Mean ± SD
81.6 ± 17.4
79.1 ± 19
0.558
0.135
-0.257–0.528
|
|
|
WOMAC Score
Preoperative
RATHA
COTHA
p-value
SMD
95% CI
End of treatment
RATHA
COTHA
p-value
SMD
95% CI
|
Mean (range)
60 (44–85)
61 (45–89)
0.517
Mean (range)
11 (6–17)
12 (5–15)
0.301
|
Mean ± SD
8.44 ±11.48
11.32 ± 11.92
0.034
-0.248
-0.759–0.264
|
|
Mean ± SD
47.07 ± 13.71
45.89 ± 10.54
0.889
Mean ± SD
4.30 ± 4.54
4.00 ± 4.27
0.875
0.068
-0.443–0.579
|
|
|
|
|
HSS: Harris Hip Score; RATHA: robotic-assisted total hip arthroplasty; COTHA: Conventional total hip arthroplasty; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; UCLA: University of California-Los Angeles.
*level of significance set at p < 0.05.
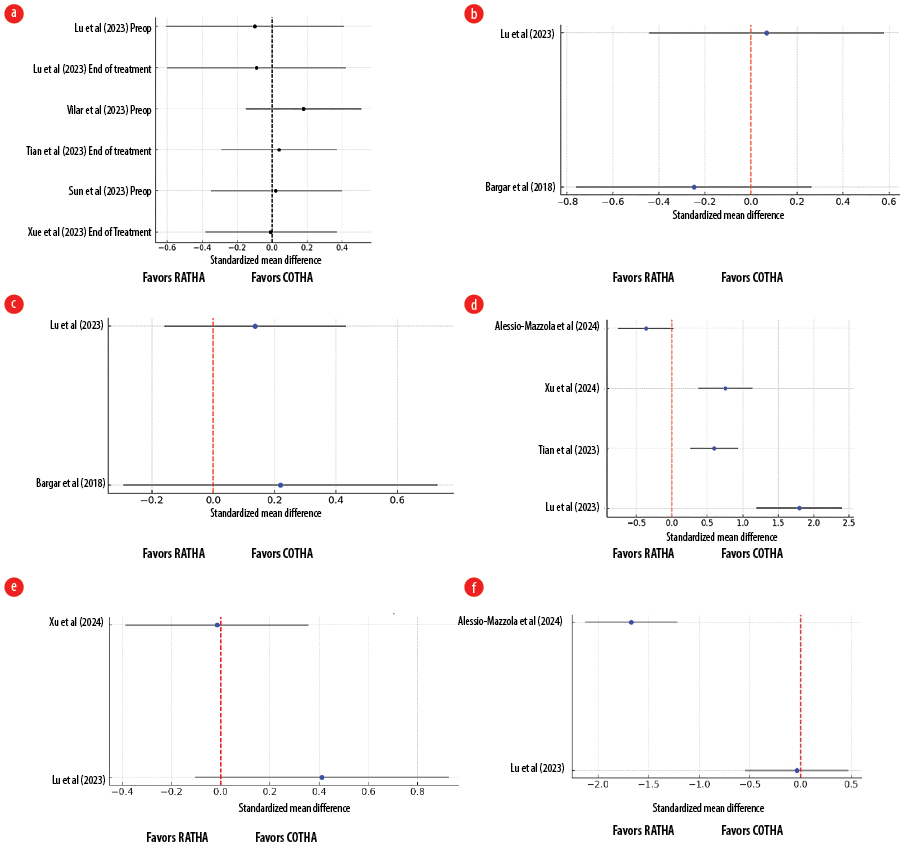 Figure 2: Forest plots showing clinical outcomes between robotic-assisted total hip arthroplasty (RATHA) and conventional total hip arthroplasty (COTHA): (a) Harris Hip Score; (b) Western Ontario and McMaster Universities Osteoarthritis Index score; (c) University of California-Los Angeles score; (d) operative times; (e) intraoperative blood loss; (f) length of stay between RATHA and COTHA.
Figure 2: Forest plots showing clinical outcomes between robotic-assisted total hip arthroplasty (RATHA) and conventional total hip arthroplasty (COTHA): (a) Harris Hip Score; (b) Western Ontario and McMaster Universities Osteoarthritis Index score; (c) University of California-Los Angeles score; (d) operative times; (e) intraoperative blood loss; (f) length of stay between RATHA and COTHA.
The secondary outcomes were operative outcomes, complications, and radiological assessment. The forest plot in Figure 2d compares the SMD in operative times between RATHA and COTHA across four studies.17–19,23 Lu et al,17 showed the largest SMD of 1.79, significantly favoring COTHA with shorter operative times. Tian et al,18 and Xu et al,19 also reported moderate SMDs (0.59 and 0.75, respectively), both favoring COTHA with statistically significant differences. However, Alessio-Mazzola et al,23 reported a small SMD (-0.36), with a CI crossing zero, indicating no significant difference between the two techniques in this study. Overall, most studies suggest that COTHA has shorter operative times compared to RATHA. The forest plot in Figure 2e compares the SMD in blood loss between RATHA and COTHA across two studies.17,19 Lu et al,17 showed a small positive SMD (0.41), suggesting that RATHA may have slightly higher blood loss than COTHA, but the CI crosses zero, indicating no statistically significant difference. Xu et al,19 reported a near-zero SMD (-0.01), with the CI also crossing zero, indicating no meaningful difference in blood loss between the two techniques. Overall, both studies suggested that there is no significant difference in blood loss between RATHA and COTHA. The forest plot in Figure 2f compares the SMD in length of stay between RATHA and COTHA across two studies.17,23 Lu et al,17 showed an SMD of -0.03, with a CI crossing zero, indicating no significant difference in length of stay between the two procedures. In contrast, Alessio-Mazzola et al,23 showed a large negative SMD of -1.67, with the CI entirely below zero, suggesting that RATHA is associated with a significantly shorter length of stay compared to COTHA. Overall, while Lu et al,17 found no difference, Alessio-Mazzola et al,23 found that RATHA reduced hospital stay duration significantly.
Table 4 presents the results of several studies comparing the rates of various complications associated with RATHA and COTHA. The studies examined revision rates, infection rates, dislocation rates, limb length discrepancies, operative times, blood loss, and lengths of stay. Overall, the results suggest no significant difference between RATHA and COTHA for most complications. Both procedures have similar rates of revision, infection, and dislocation. Additionally, there is no significant difference in limb length discrepancy between the two groups.
Table 4: Summary of revision rates, infection, dislocation, limb length discrepancy, blood loss intraoperatively, length of stay and operative time of included trials.
|
Revision
RATHA
COTHA
|
0/24
2/25
|
4/45
6/22
|
0/64
0/64
|
|
|
|
|
|
Infection
RATHA
COTHA
p-value
|
0/24
0/25
1.000
|
0/45
0/22
|
0/30
0/29
|
|
|
|
2/50
1/50
0.594
|
|
Dislocation
RATHA
COTHA
p-value
|
0/24
0/25
1.000
|
1/45
0/22
|
|
|
|
|
0/50
0/50
1.000
|
|
Limb Length Discrepancy, mm
RATHA
COTHA
p-value
SMD
95% CI
|
Mean (range)
1.9 (0–6.4)
4.9 (0–16)
0.011*
|
|
|
|
Mean ± SD
6.0 ± 5.0
8.09 ± 4.33
0.000*
-0.448
-0.772–-0.124
|
Mean ± SD
2.27 ± 4.19
1.29 ± 4.33
0.2538
0.229
-0.102–0.56
|
Mean ± SD
0.6 ± 1.4
0.4 ± 1.4
0.572
0.143
-0.251–0.537
|
|
Blood loss, mL
RATHA
COTHA
p-value
SMD
95% CI
|
Mean (range)
1010 (610–1800)
895 (410–1370)
0.271
|
|
|
Mean ± SD
1280.30 ± 404.01
1094.86 ± 494.39
0.137
0.411
-0.104–0.927
|
|
Mean ± SD
163.8 ± 118.5
165.5 ± 113.4
0.9384
-0.015
-0.387–0.357
|
|
|
Length of stay, days
RATHA
COTHA
p-value
SMD
95% CI
|
|
|
|
Mean ± SD
5.29 ± 0.53
5.31 ± 0.54
0.863
-0.037
-0.548–0.473
|
|
|
Mean±SD
2.5±0.6
4.3±1.4
< 0.001*
-1.671
-2.127–-1.216
|
RATHA: robotic-assisted total hip arthroplasty; COTHA: conventional total hip arthroplasty; SMD: standardized mean differences.
*level of significance set at p < 0.05.
Table 5 presents the results of several studies comparing the radiological outcomes of RATHA and COTHA.15,18,19,23 The studies examined femoral stem alignment outliers, stem appropriate size, cup malalignment, Lewinnek safe zone, and global offset. Overall, the results suggest no significant difference between RATHA and COTHA for most radiological outcomes. Both procedures have similar rates of femoral stem alignment outliers, cup malalignment, and global offset. However, there are some minor differences.
Table 5: Summary of radiological outcomes of included trials.
|
Femoral stem alignment Outlier (> ± 3o)
RATHA
COTHA
p-value
|
0/24
6/25
0.022*
|
|
|
0/50
1/50
0.485
|
|
Cup malalignment
RATHA
COTHA
p-value
|
|
|
|
0/50
2/50
0.141
|
|
Stem appropriate size
RATHA
COTHA
p-value
|
|
57 (90.5%)
69 (86.3%)
0.438
|
|
48 (96.0%)
49 (98.0%)
1.000
|
|
Lewinnek safe zone
RATHA
COTHA
p-value
|
|
57 (90.5%)
62 (77.5%)
0.039*
|
52 (92.9%)
47 (85.5%)
0.2092
|
|
RATHA: robotic-assisted total hip arthroplasty; COTHA: conventional total hip arthroplasty. *level of significance set at p < 0.05.
The stem appropriate size was slightly higher for RATHA patients in Tian et al,18 but there was no significant difference in Alessio-Mazzola et al.23 The Lewinnek safe zone was also slightly higher for RATHA patients in both studies.
Discussion
This review highlights that RATHA and COTHA yield comparable clinical outcomes in terms of PROMs, with no significant differences in complications, a finding consistently reported in previous systematic reviews.24–27 COTHA demonstrated shorter operative times, reflecting greater procedural efficiency, as has been well-documented in previous studies.16,24–27 In contrast, RATHA offers a distinct advantage in radiological precision, particularly in achieving more accurate implant alignment, which may confer long-term benefits. Systematic reviews by Kumar et al,25 and Han et al,26 similarly concluded that RATHA results in superior implant placement accuracy, which is consistent with the findings of this review.
Previous systematic reviews comparing RATHA and COTHA often included older trials, some dating back to 1998.24–26 The current literature comparing RATHA and COTHA is scarce and low-quality, with findings limited by methodological flaws in study design.27 The global prevalence of OA has grown from 247.5 million cases in 1990 to 527.8 million in 2019, representing an increase of 113.3% over three decades.28 OA is the most common cause of arthritis worldwide, being almost a universal problem in individual aged ≥ 65 years.29 There is a growing need to understand the benefits of robotic joint arthroplasty properly. This systematic review evaluated the comparative performance of RATHA and COTHA across clinical and radiological outcomes using studies published in the last 10 years. Primary outcomes were clinical outcomes measured using PROMs, which included HHS, WOMAC, and UCLA scores. The secondary outcomes were operative outcomes, complications, and radiological assessment. This included operative characteristics (operative time, blood loss, and length of hospital stay)and radiological precision (femoral stem alignment, cup malalignment, stem appropriateness, Lewinnek safe zone compliance, and global offset).
The clinical outcomes assessed through the HHS, WOMAC, and UCLA scores revealed no significant differences between RATHA and COTHA. Across multiple studies, the SMDs were small, and the CI crossed zero, indicating that both techniques are equally effective in improving hip function, reducing pain, and maintaining activity levels. Although RATHA offers more precision in implant placement, as highlighted in the radiological outcomes, these advantages did not translate into significant differences in clinical function or pain relief, as measured by these scores.
RATHA and COTHA showed distinct differences in operative outcomes. COTHA consistently demonstrated shorter operative times across multiple studies, with significant SMDs favoring the conventional technique. This suggests that COTHA remains a faster and potentially more efficient option. On the other hand, RATHA showed a potential benefit in reducing the length of hospital stay, as reported by Alessio-Mazzola et al,23 although other studies showed no difference. Blood loss, however, showed no consistent difference between the two techniques, indicating that both approaches are comparable in this aspect of surgical management.
RATHA demonstrated notable advantages in several radiological outcomes, suggesting greater precision in implant positioning. Studies have shown that RATHA is associated with fewer femoral stem alignment outliers and better compliance with the Lewinnek safe zone, which are key indicators of proper implant alignment. Specifically, Lim et al,15 and Tian et al,18 highlighted the superiority of RATHA in achieving more accurate implant placement. These radiological differences suggest that RATHA may offer long-term benefits in terms of reducing complications such as implant malalignment or dislocation; however, the clinical implications of these findings were not fully realized in the PROMs.
Robotic systems require significant initial capital expenditure and ongoing maintenance, which can strain healthcare budgets, especially in resource-limited settings. RATHA’s radiological precision may reduce the likelihood of revision surgeries or long-term complications, particularly benefiting younger, more active patients or those with complex anatomy. However, for older or lower-demand patients, where the emphasis is on immediate functional recovery and cost containment, COTHA remains an efficient and effective choice. While RATHA provides enhanced precision in implant placement, its clinical advantages over COTHA remain limited in the short to medium term, as evidenced by similar functional outcomes and operative efficiency favoring COTHA.
The risk of bias assessment using the Cochrane Risk of Bias Tool revealed variability in the methodological rigor of the studies. While several studies employed adequate randomization methods and ensured blinding of outcome assessors,17,19 other studies lacked sufficient details on randomization and blinding procedures, introducing potential for selection and performance bias.15,20 Furthermore, the non-randomized nature of studies heightened the risk of bias due to the lack of random sequence generation and allocation concealment.21,22 The retrospective design in some studies also limited the strength of evidence, as recognized in Tian et al,18 where the small sample size and lack of blinding may have influenced the results. Despite these limitations, the overall risk of selective reporting appeared low, as most studies provided comprehensive outcome data.
The studies included in this review had several strengths and limitations that shaped our findings. One notable strength is the diversity of study designs, including RCTs, retrospective cohort studies, and prospective analyses. This diversity has enable a comprehensive evaluation of clinical and radiological outcomes across different healthcare settings and patient populations. Most studies have been published within the last decade, ensuring relevance to current surgical practices and technologies, particularly RATHA. Furthermore, the international representation of studies, with contributions from Asia, the USA, the UK, and Italy, enhances the generalizability of the findings across various populations and healthcare systems. Robust outcome measures were another strength, with studies employing validated metrics, such as the HHS, WOMAC, and UCLA scores for clinical outcomes, along with specific radiological metrics, such as femoral stem alignment and compliance with the Lewinnek safe zone. This multidimensional approach provided a comprehensive evaluation of the performance of RATHA and COTHA. Additionally, a rigorous quality assessment using the Cochrane Risk of Bias Tool helped identify methodological strengths and weaknesses, ensuring transparency and reliability in the interpretation of the results. The review also emphasized RATHA’s radiological precision, highlighting its potential long-term benefits, such as reduced complications and improved implant longevity.
Despite these strengths, this study had several limitations. The scarcity of high-quality RCTs is a significant drawback that reduces the ability to draw robust causal inferences. Nonrandomized designs, such as retrospective studies, are inherently more susceptible to bias and confounding factors. Additionally, the studies displayed significant heterogeneity in design, patient population, surgical techniques, robotic systems, and outcome measures, making data synthesis and comparison challenging. Many studies also lacked details on blinding and randomization procedures, increasing the risk of selection and performance bias. Small sample sizes and short follow-up durations are common, limiting the ability to evaluate long-term outcomes, such as implant survival and late complications. The potential for conflicts of interest was another concern, as some studies involved robotic systems developed by commercial entities that were not always disclosed or addressed transparently. Complications such as blood loss, infection rates, and revision rates have been inconsistently reported, potentially underestimating the associated risks. Moreover, the variability in the robotic platforms used, such as the ROBODOC, Mako, and ROSA systems, introduced technological inconsistencies, complicating the generalization of findings to all robotic-assisted surgeries. The review also excluded non-English studies, potentially omitting valuable data and introducing a language bias. Furthermore, reliance on published studies may have led to publication bias, as negative or inconclusive results are less likely to be reported. Finally, the studies focused more on short- and medium-term outcomes, with limited emphasis on long-term results, such as implant durability and patient satisfaction over time.
To mitigate these limitations, this review explicitly acknowledged the inclusion of non-RCTs and addressed their impact through transparent reporting and consistency analysis. A thorough risk of bias assessment highlighted potential methodological flaws, ensuring critical evaluation of the findings. The emphasis on consistent trends across studies reinforced the reliability of conclusions despite the variability in study designs. These mitigation strategies underscore the evolving nature of evidence in the field of RATHA and highlight the need for high-quality, large-scale, and long-term RCTs to validate the findings.
Conclusion
The comparison between RATHA and COTHA demonstrated that both techniques yielded similar clinical outcomes in terms of functional recovery and pain relief. However, RATHA shows a clear advantage in radiological precision, particularly in achieving a more accurate implant alignment, which could have long-term benefits. In contrast, COTHA was associated with shorter operative times, indicating greater procedural efficiency. While RATHA’s precision may have benefits in specific clinical contexts, the decision between these two techniques should be tailored to the patient’s needs, the surgeon’s expertise, and available resources. The cost-effectiveness of RATHA depends on the long-term realization of its radiological benefits. The risk of bias assessment highlights the need for more rigorous and well-reported RCTs to better assess the comparative efficacy of these two techniques. Future large-scale, long-term studies are needed to evaluate whether RATHA’s precision yields substantial clinical and economic benefits over time, guiding its integration into clinical practice.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Fu M, Zhou H, Li Y, Jin H, Liu X. Global, regional, and national burdens of hip osteoarthritis from 1990 to 2019: estimates from the 2019 global burden of disease study. Arthritis Res Ther 2022 Jan;24(1):8.
- 2. Matharu GS, Culliford DJ, Blom AW, Judge A. Projections for primary hip and knee replacement surgery up to the year 2060: an analysis based on data from the national joint registry for England, Wales, Northern Ireland and the Isle of Man. Ann R Coll Surg Engl 2022 Jun;104(6):443-448.
- 3. Population and Housing Census. Key findings population and housing census of Malaysia 2020: administrative district. 2022 [cited 2023 August 1]. Available from: https://www.dosm.gov.my/portal-main/release-content/key-findings-population-and-housing-census-of-malaysia-2020-administrative-district.
- 4. Banerjee S, Cherian JJ, Elmallah RK, Pierce TP, Jauregui JJ, Mont MA. Robot-assisted total hip arthroplasty. Expert Rev Med Devices 2016;13(1):47-56.
- 5. Yu F, Li L, Teng HJ, Shi DQ, Jiang Q. Robots in orthopedic surgery. Ann Joint 2018;3(3).
- 6. Tsai TY, Li JS, Chang WN, Lin CJ, Hsieh PH, Lee MS. Comparison of clinical outcomes between computer-assisted navigation and conventional instrumentation in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 2023;18(1):45.
- 7. Bloem RM, Mak JN, Schreurs BW, Hannink G. Long-term outcomes of impaction bone grafting in revision total hip arthroplasty: a 20-year follow-up study. Bone Joint J 2022;104-B(2):150-156.
- 8. Mont MA, Nunley RM, Della Valle CJ, Barrack RL, Clohisy JC. Management of metal-on-metal hip implant patients: a multicenter study. Orthop Traumatol Surg Res 2023;109(1):104875.
- 9. Chen AF, Haddad FS, Lachiewicz PF, Springer BD. Prevention of periprosthetic joint infection: the role of perioperative antibiotics. J Arthroplasty 2023;38(1):S25-S29.
- 10. Llombart-Blanco R, Mariscal G, Barrios C, Vera P, Llombart-Ais R. MAKO robot-assisted total hip arthroplasty: a comprehensive meta-analysis of efficacy and safety outcomes. J Orthop Surg Res 2024 Oct;19(1):698.
- 11. Kim K, Kwon S, Kwon J, Hwang J. A review of robotic-assisted total hip arthroplasty. Biomed Eng Lett 2023 Aug;13(4):523-535.
- 12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021 Mar;372(71):n71.
- 13.Higgins JP. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. The Cochrane Collaboration. BMJ 2011;343:d5928.
- 14. Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia. 2017;680 [cited 2023 August 1]. Available from: https://app.covidence.org/reviews/486502
- 15. Lim S-J, Ko K-R, Park C-W, Moon Y-W, Park Y-S. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg 2015;20(1):41-46.
- 16. Nakamura N, Sugano N, Sakai T, Nakahara I. Does robotic milling for stem implantation in cementless THA result in improved outcomes scores or survivorship compared with hand rasping? Results of a randomized trial at 10 years. Clin Orthop Relat Res 2018 Nov;476(11):2169-2173.
- 17. Lu H, Sun H, Xiao Q, Xu H, Zhou Q, Li L, et al. Perioperative safety and efficacy of robot-assisted total hip arthroplasty in ERAS-managed patients: a pilot study. J Orthop Surg Res 2023 Sep;18(1):696.
- 18. Tian R, Duan X, Kong N, Wang K, Yang P. Precise acetabular positioning, discrepancy in leg length, and hip offset using a new seven-axis robot-assisted total hip arthroplasty system requires no learning curve: a retrospective study. J Orthop Surg Res 2023 Mar;18(1):236.
- 19. Xu Z, Chai S, Chen D, Wang W, Dai J, Zhang X, et al. The LANCET robotic system can improve surgical efficiency in total hip arthroplasty: a prospective randomized, multicenter, parallel-controlled clinical trial. J Orthop Translat 2024 Apr;45:247-255.
- 20. Bargar WL, Parise CA, Hankins A, Marlen NA, Campanelli V, Netravali NA. Fourteen year follow-up of randomized clinical trials of active robotic-assisted total hip arthroplasty. J Arthroplasty 2018 Mar;33(3):810-814.
- 21. Buchan GB, Ong CB, Hecht Ii CJ, DeCook CA, Spencer-Gardner LS, Kamath AF. Use of a fluoroscopy-based robotic-assisted total hip arthroplasty system produced greater improvements in patient-reported outcomes at one year compared to manual, fluoroscopic-assisted technique. Arch Orthop Trauma Surg 2024 Apr;144(4):1843-1850.
- 22. Fontalis A, Kayani B, Haddad IC, Donovan C, Tahmassebi J, Haddad FS. Patient-reported outcome measures in conventional total hip arthroplasty versus robotic-arm assisted arthroplasty: a prospective cohort study with minimum 3 years’ follow-up. J Arthroplasty 2023 Jul;38(7)(Suppl 2):S324-S329.
- 23. Alessio-Mazzola M, Colombo P, Barducci N, Ghezzi E, Zagra L, Caldora P, et al. Direct anterior approach with conventional instruments versus robotic posterolateral approach in elective total hip replacement for primary osteoarthritis: a case-control study. J Orthop Traumatol 2024 Feb;25(1):9.
- 24. Ruangsomboon P, Ruangsomboon O, Osman K, Pincus D, Mundi R, Tomescu S, et al. Clinical, functional, and radiological outcomes of robotic assisted versus conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Robot Surg 2024 Jun;18(1):255.
- 25. Kumar V, Patel S, Baburaj V, Rajnish RK, Aggarwal S. Does robotic-assisted surgery improve outcomes of total hip arthroplasty compared to manual technique? A systematic review and meta-analysis. Postgrad Med J 2023 Jun;99(1171):375-383.
- 26. Han PF, Chen CL, Zhang ZL, Han YC, Wei L, Li PC, et al. Robotics-assisted versus conventional manual approaches for total hip arthroplasty: a systematic review and meta-analysis of comparative studies. Int J Med Robot 2019 Jun;15(3):e1990.
- 27. Sweet MC, Borrelli GJ, Manawar SS, Miladore N. Comparison of outcomes after robotic-assisted or conventional total hip arthroplasty at a minimum 2-year follow-up: a systematic review. JBJS Rev 2021 Jun;9(6):e20.
- 28. Singh S, Singh S, Nachimuthu M, Kassim AF, Bhullar AK, Veerakumaran R, et al. Effectiveness of ultrasound-guided versus anatomical landmark-guided genicular nerve block to treat chronic knee osteoarthritis: a retrospective cohort study. Oman Med J 2023 Sep;38(5):e550.
- 29. Nik Shafii NA, Yaacob LH, Ishak A, Kadir AA. Traditional and complementary medicine use in knee osteoarthritis and its associated factors among patients in Northeast Peninsular Malaysia. Oman Med J 2018 Mar;33(2):148-153.