Emphysematous pyelonephritis (EPN) is a rare necrotizing infection of the renal parenchyma and pelvicalyceal system involving gas-forming pathogens such as Escherichia coli and Klebsiella pneumoniae. Typical risk factors include poorly controlled diabetes mellitus (DM), immunosuppressed states, and urinary tract obstruction.1–3 Due to the risk of acute renal failure and life-threatening sepsis, patients with EPN require prompt diagnosis and treatment.2
In diabetic patients, urinary tract infections (UTIs) are common and can range from asymptomatic bacteriuria to severe urosepsis, emphysematous pyelonephritis orcystitis, and renal abscess formation.4 Diabetic patients with poor glycemic control tend to have impaired humoral and cell-mediated immunity, with higher susceptibility to opportunistic infections.5
Commensal bacteria such as Bifidobacterium species are generally considered safe and beneficial even for low-immunity patients.5 We present a novel case where Bifidobacterium breve was identified as a pathogen causing polymicrobial EPN in an immunocompromised patient.
Case Report
The patient was a 71-year-old Chinese woman, with a history of poorly controlled DM on insulin therapy, and end-stage renal failure (ESRF) managed by peritoneal dialysis (PD). She presented with a two-day history of gross hematuria, dysuria, and fever, without abdominal/flank pain. On examination, she was hemodynamically stable and afebrile, with a soft and non-tender abdomen and negative bilateral renal angle tenderness. Initial laboratory tests revealed leukocytosis and elevated C-reactive protein. Urinalysis demonstrated significant pyuria and hematuria, and urine culture grew Klebsiella sp. Blood and PD fluid cultures were negative for pathogens [Table 1]. Computed tomography scan of the abdomen and pelvis revealed bilateral renal abscesses with gas locules within the right renal parenchyma and pelvicalyceal system [Figure 1], consistent with Huang and Tseng class II emphysematous pyelonephritis.6
Table 1: Results of laboratory and microbiological investigations on admission.
|
Test
|
|
|
White blood cells, /L
|
18.2 × 109
|
4–10 × 109
|
|
Hemoglobin, g/dL
|
13.4
|
12–16
|
|
Platelets, /L
|
162 × 109
|
140–440
|
|
Sodium, mmol/L
|
129
|
136–146
|
|
Potassium, mmol/L
|
3.5
|
3.6–5.0
|
|
Creatinine, umol/L
|
646
|
37–75
|
|
Urea, mmol/L
|
9.9
|
2.7–6.9
|
|
Bicarbonate, mmol/L
|
25.2
|
19–29
|
|
Glucose, mmol/L
|
18.3
|
3.9–11.0
|
|
Calcium (corrected), mmol/L
|
2.25
|
2.09–2.46
|
|
Magnesium, mmol/L
|
0.52
|
0.74–0.97
|
|
Phosphate, mmol/L
|
1.62
|
0.9–1.5
|
|
C-reactive protein, mg/dL
|
293
|
0.2–9.1
|
|
Lactate, mmol/L
|
3.5
|
0.5–2.2
|
|
HbA1c, %
|
9.3
|
< 7
|
|
Microbiology
|
|
Blood culture (aerobic)
|
No bacterial growth
|
|
Blood culture (anaerobic)
|
No bacterial growth
|
|
Urine, full examination, and microscopic examination
|
> 2000 WBCs
|
|
> 2000 RBCs
|
|
0 epithelial cells
|
|
Urine culture
|
Klebsiella spp.
|
|
Peritoneal fluid cell count
|
0
|
|
Peritoneal fluid culture
|
No bacterial growth
|
|
Tissue gram stain smear
|
Gram-positive bacilli 2+
|
|
Polymorphs 3+
|
|
Tissue culture
|
1. Bifidobacterium breve
|
|
2. Klebsiella spp. – Sensitive to amoxicillin-clavulanic acid, piperacillin-tazobactam, ceftriaxone, cefepime, aztreonam, ertapenem, gentamicin, and ciprofloxacin. Resistant to ampicillin
|
|
Tissue culture for Nocardia
|
Negative
|
|
Tissue acid fast bacilli smear and culture
|
Negative
|
|
Tissue mycobacterium tuberculosis PCR
|
Negative
|
|
Tissue fungal microscopy and culture
|
Negative
|
HbA1c: glycated hemoglobin; WBC: white blood cell; RBC: red blood cells; PCR: polymerase chain reaction.
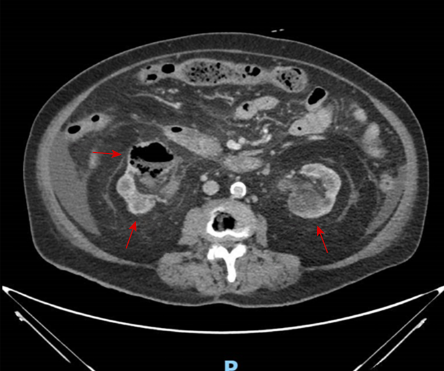 Figure 1: Contrast-enhanced CT image of the abdomen and pelvis showing bilateral renal abscesses (red arrows) and right-sided emphysematous pyelonephritis. Dashed arrow indicates the location of gas within the right renal pelvicalyceal system.
Figure 1: Contrast-enhanced CT image of the abdomen and pelvis showing bilateral renal abscesses (red arrows) and right-sided emphysematous pyelonephritis. Dashed arrow indicates the location of gas within the right renal pelvicalyceal system.
The patient was initially treated with empirical intravenous (IV) aztreonam, as she was allergic to ceftriaxone. Percutaneous drainage of the right-sided gas-forming infected renal collections was performed for source control. Gram stain of the drained sample revealed gram-positive bacilli. Tissue cultures yielded significant growth of both Klebsiella spp. and B. breve. As the patient continued to have fevers despite treatment with aztreonam, IV vancomycin was added to cover for Bifidobacterium spp. Thereafter, her condition improved rapidly, with a resolution of fever and a downtrend in inflammatory markers. She completed five weeks of antibiotic therapy to resolve her polymicrobial EPN, comprising one week of IV aztreonam followed by four weeks of oral ciprofloxacin, and five weeks of IV vancomycin. A second computed tomography scan of the abdomen and pelvis performed toward the end of her antibiotic course also demonstrated the resolution of the infective renal collections. Written consent for publication of this case was obtained from the patient.
Discussion
EPN is a severe gas-forming infection of the renal parenchyma and collecting system that can lead to acute renal failure, septicemia, and death. The most common causative organisms usually include gram-negative bacteria, Escherichia coli, and Klebsiella pneumoniae, with less frequently cases involving Proteus, Pseudomonas, Streptococcus, anaerobes, fungi, and occasionally polymicrobial infections.1,2 Classical risk factors described in literature include poorly controlled DM, immunosuppression, and urinary tract obstruction.1,2 Major contributors to the pathogenesis of EPN include poor glycemic control associated with impaired renal vascular supply and host leukocyte function, immunodeficiency, the presence of gas-forming microorganisms, and urinary stasis due to structural urinary tract obstruction.1,2,7 Management of EPN is commonly guided by radiological staging of the disease, as described by Huang and Tseng,6 and consists of systemic antimicrobial therapy, percutaneous drainage of renal abscess, and/or surgical nephrectomy.1 To our knowledge, we reported the first documented case of polymicrobial EPN involving K. pneumoniae and B. breve in a diabetic patient on PD.
B. breve is a highly unusual cause of emphysematous pyelonephritis, as Bifidobacterium spp. are typically known as harmless and beneficial components of the human gut microbiota.8 However, there have been rare reports of Bifidobacteria becoming pathogenic in immunocompromised hosts, likely due to gut bacterial translocation.9 UTIs involving Bifidobacterium spp. are extremely rare, with only two recent reports. The first was a case of polymicrobial UTI involving Candida glabrata and Bifidobacterium in a patient with uncontrolled diabetes and myelodysplastic syndrome.10 The second case was one of recurrent UTI associated with B. scarovii in an immunocompromised patient receiving corticosteroids for autoimmune hemolytic anemia with a previous history of breast cancer treated with chemotherapy and radiotherapy.11
Our patient’s history of poorly controlled DM and ESRF on PD may have caused gut bacterial translocation, leading to an opportunistic infection by B. breve. It is reported that hyperglycemia may increase the permeability of the intestinal epithelial barrier through glucose transporter 2-mediated mechanisms, promoting gut bacterial translocation.12 In ESRF patients, impaired immunity is often related to the accumulation of uremic toxins following impaired kidney function.13 Moreover, ESRF is also associated with gut bacterial translocation, which may lead to microinflammation.14 A recent UTI microbiome cohort study found that a dysbiotic gut microbiome may predispose females to recurrent UTIs,15 consistent with a recently proposed theory that the gut microbiota might actually be the main contributing source of UTIs.16 Additionally, the high dextrose content in peritoneal dialysates can worsen glycemic control in diabetic patients and even inflict damage to the peritoneal membranes.17 Isolated case reports have documented emphysematous pyelonephritis18 and pyelitis19 in patients on PD, although further studies are needed to establish if there is a significant clinical association.
Finally, Bifidobacterium spp. isolated from sterile tissue specimens should not simply be disregarded as a contaminant.10 In particular, in immunocompromised patients with a local or systemic infection, where Bifidobacterium has been isolated from a potential site of infection and the patient has not adequately responded to empirical antimicrobial treatment, it may be prudent to include antibiotic cover for Bifidobacterium spp. with β-lactams, vancomycin, or clindamycin.9,20 In addition, for species such as B. breve, which is associated with greater antimicrobial resistance,20 anaerobic susceptibility testing may also have a role, although it is not routinely performed in microbiological laboratories, should there be inadequate clinical response to standard antimicrobial treatment.
Conclusion
We have described a novel case of polymicrobial emphysematous pyelonephritis involving the anaerobic gut commensal B. breve as one of the causative pathogens and highlighted several learning points from this clinical vignette for the management of similar cases in future.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Pontin AR, Barnes RD. Current management of emphysematous pyelonephritis. Nat Rev Urol 2009 May;6(5):272-279.
- 2. Ubee SS, McGlynn L, Fordham M. Emphysematous pyelonephritis. BJU Int 2011 May;107(9):1474-1478.
- 3. Azfar SF, Badar F, Akhtar N, Kirmani S. Diabetic women with fever and right flank pain. Oman Med J 2011 Mar;26(2):141-142.
- 4. Akash MS, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol 2020 Jul;202(5):953-965.
- 5. Khanam A, Hithamani G, Naveen J, Pradeep SR, Barman S, Srinivasan K. Management of invasive infections in diabetes mellitus: a comprehensive review. Biologics 2023;3(1):40-71.
- 6. Huang J-J, Tseng C-C. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med 2000 Mar;160(6):797-805.
- 7. Sokhal AK, Kumar M, Purkait B, Jhanwar A, Singh K, Bansal A, et al. Emphysematous pyelonephritis: changing trend of clinical spectrum, pathogenesis, management and outcome. Turk J Urol 2017 Jun;43(2):202-209.
- 8. O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 2016 Jun;7:925.
- 9. Esaiassen E, Hjerde E, Cavanagh JP, Simonsen GS, Klingenberg C; Norwegian Study Group on Invasive Bifidobacterial Infections. Bifidobacterium bacteremia: clinical characteristics and a genomic approach to assess pathogenicity. J Clin Microbiol 2017 Jul;55(7):2234-2248.
- 10. Pathak P, Trilligan C, Rapose A. Bifidobacterium–friend or foe? A case of urinary tract infection with Bifidobacterium species. BMJ Case Rep 2014 Sep;2014:bcr2014205122.
- 11. Barberis CM, Cittadini RM, Almuzara MN, Feinsilberg A, Famiglietti AM, Ramírez MS, et al. Recurrent urinary infection with Bifidobacterium scardovii. J Clin Microbiol 2012 Mar;50(3):1086-1088.
- 12. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol 2020 Jul;11:1582.
- 13. Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr 2012 Jan;22(1):149-156.
- 14. Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 2012 Nov;17(8):733-738.
- 15. Worby CJ, Schreiber HL IV, Straub TJ, van Dijk LR, Bronson RA, Olson BS, et al. Longitudinal multi-omics analyses link gut microbiome dysbiosis with recurrent urinary tract infections in women. Nat Microbiol 2022 May;7(5):630-639.
- 16. Stepanova N. How advanced is our understanding of the role of intestinal barrier dysfunction in the pathogenesis of recurrent urinary tract infections. Front Pharmacol 2022 Mar;13:780122.
- 17. Lee MJ, Kwon YE, Park KS, Kee YK, Yoon CY, Han IM, et al. Glycemic control modifies difference in mortality risk between hemodialysis and peritoneal dialysis in incident dialysis patients with diabetes: results from a nationwide prospective cohort in Korea. Medicine (Baltimore) 2016 Mar;95(11):e3118.
- 18. Borrajo Prol MP, Pérez Melón C, Santos Nores J, Camba Caride M. [Emphysematous pyelonephritis in peritoneal dialysis]. Nefrologia 2008;28(6):663-664.
- 19. Anwar N, Chawla LS, Lew SQ. Emphysematous pyelitis presenting as an acute abdomen in an end-stage renal disease patient treated with peritoneal dialysis. Am J Kidney Dis 2002 Oct;40(4):E13.
- 20. Moubareck C, Gavini F, Vaugien L, Butel MJ, Doucet-Populaire F. Antimicrobial susceptibility of bifidobacteria. J Antimicrob Chemother 2005 Jan;55(1):38-44.