Geriatric medicine is a relatively new specialty, recognized over the past two decades in response to the increasing healthcare needs of an aging population. Among the myriad health concerns that accompany advanced age, generalized weakness stands out as a common and often perplexing symptom.1,2 The challenges posed by an aging population are complex and multifaceted, evolving over centuries and unlikely to diminish suddenly. Generalized weakness in geriatric patients is multifactorial, often involving several geriatric syndromes and various etiological factors such as comorbid conditions, poor physical health, malnutrition, anemia, electrolyte imbalances, drug-induced myasthenia, deconditioning, coexistent depression, acute/chronic infections, urinary tract infections, chronic cerebrovascular diseases, and patient-specific issues such as immobilization, dehydration, and pain, even after excluding causes related to sarcopenia.1,3-5 In the emergency department (ED), geriatric patients frequently present with a chief complaint of generalized weakness without a clear precipitant, which complicates diagnostic approaches. Prompt identification of the underlying cause is crucial, as failure to detect acute life-threatening disorders could prove fatal.
Chew and Birnbaumer's 1999 recommendations emphasize guiding the physical examination by the patient’s medical history and comorbidities, with sensitivity to geriatric issues, including social history.6 The neurologic examination is crucial to determine if symptoms are focal or non-focal. Diagnosing the underlying pathology for generalized weakness is complex, often requiring exhaustive evaluation through comprehensive history-taking, physical examinations, and laboratory and radiological investigations.6,7 Brain computed tomography (CT) scans are commonly performed to evaluate these patients for underlying pathologies. In the complex diagnostic process, CT brain imaging stands out for its clarity and accuracy. Although generalized weakness does not always indicate intracranial pathology, CT scans are essential for ruling out serious conditions such as acute/ subacute cerebrovascular accidents, or other space-occupying lesions (SOLs).8,9 CT brain imaging also aids in distinguishing between central and peripheral causes of generalized weakness. These conditions often present with vague symptoms like weakness, changes in mental state, or other atypical manifestations. The primary goal of performing CT brain scans in elderly patients with generalized weakness is to promptly identify urgent issues requiring immediate attention, such as acute/subacute ischemic stroke or hemorrhagic stroke, which can manifest as weakness.8,9 Early detection is critical for initiating timely treatments such as thrombolysis (within 4.5 hr), antiplatelet drugs, surgery, or specialized neurological care, thereby reducing the risk of severe outcomes and improving patient health.
However, concerns about the investigation's high cost, potential radiation hazard, and the allocation of hospital resources make its use controversial. Our study aimed to determine the role of CT scan in this age group and its significance. This study is one of the first from Oman involving the EDs of two large academic centers and will provide valuable insights to the global literature.
Methods
This retrospective cross-sectional cohort study used patient data from two academic tertiary care hospitals in Muscat, Oman. Each ED within these hospitals holds platinum-level recognition and handles over 10 000 geriatric patients annually, encompassing both medical and surgical cases. The facilities include 24-hour CT scaning and continuous on-site radiologist support for expert result interpretation.
We sought to assess the diagnostic effectiveness of CT scans in elderly patients presenting with generalized weakness. Data were reviewed for elderly patients who underwent CT brain scans due to weakness over a one-year period (1 January – 31 December 2022). Objectives included profiling clinical characteristics, vital signs, sensorium, and metabolic parameters to identify underlying causes. Additionally, the study examined ED disposition and subsequent hospital outcomes for these patients. Elderly patients (age ≥ 65 years) presenting to the ED with generalized weakness or related symptoms such as fatigue, dizziness, vertigo, fever, nausea, or vomiting were included. Individuals with recent trauma, acute stroke symptoms, or specific neurological conditions like migraines, or those known to have a space-occupying lesion in the brain or metastasis were excluded from the study. Medical charts lacking essential data were also excluded.
Calhoun et al,10 found a 3.2% prevalence of acute intracranial abnormalities on CT brain imaging in elderly patients with generalized weakness. To achieve a precision of 1.2% and a 95% CI, approximately 829 patients were needed for the study. Clinical data, including age, sex, presenting complaints, vital signs, laboratory results, and radiological findings (e.g., chest radiographs), were entered into a standardized data collection sheet. The primary investigator supervised the process, and an interim analysis was conducted to minimize data entry errors.
Data were extracted from the hospital's electronic records and analyzed using IBM SPSS Statistics (IBM Corp. Release 2022. IBM SPSS Statistics for Windows, Version 29.0 Armonk, NY: IBM Corp.). Quantitative variables were summarized using mean and SD, while qualitative variables were reported as frequencies and percentages. Univariate and bivariate logistic regression analyses were conducted, with significance defined as p < 0.05. We plotted receiver operating characteristic (ROC) curves to assess the CT scan discriminatory power, specifically analyzing its ability to detect significant new or acute CT findings (e.g., acute or acute-on-chronic subdural hematomas, acute or subacute ischemic cerebrovascular accidents, and newly detected SOLs).
The research proposal received ethical approval from the Medical Research and Ethics Committees of both institutes. Approval was granted by Sultan Qaboos Medical University (REF. NO. SQU-EC/ 285\2023MREC # 3182 dated 3 December 2023) and the Royal Hospital (Ref no: MoH/CSR/23/27710 dated 9 January 2024). Ethical guidelines were adhered to, ensuring patient confidentiality through measures such as unique identity documentation and password-protected data entry restricted to authorized personnel.
Results
The total number of elderly patients who visited the ED of the two hospitals was 12 608, only 847 (6.7%) out of 965 presented with generalized weakness and underwent a CT scan of the brain as part of their evaluation [Figure 1]. The mean age was 76.0 ± 7.7 years, with males comprising 47.5% of the group. Common comorbidities included hypertension (71.4%), diabetes mellitus (52.5%), and dyslipidemia (38.4%), alongside prevalent conditions such as ischemic heart disease (35.1%), chronic kidney disease (20.3%), and cerebrovascular accident (7.7%)
[Table 1]. Presenting complaints included fever (57.0%), drowsiness (46.9%), dizziness (30.0%), headache (24.0%), and other symptoms [Table 2]. Abnormal vital signs were noted in a significant number of cases, including tachycardia (21.2%), tachypnea (27.0%), and extremes of blood pressure readings. Radiological findings revealed acute ischemic changes (3.3%), subacute ischemic changes (7.8%), acute on chronic subdural hematoma or intracranial hematoma (3.4%), new SOLs or abscesses (4.4%), and other unspecified abnormalities (6.1%) [Table 3]. Laboratory results showed abnormal markers, including electrolyte imbalances and elevated inflammatory markers.
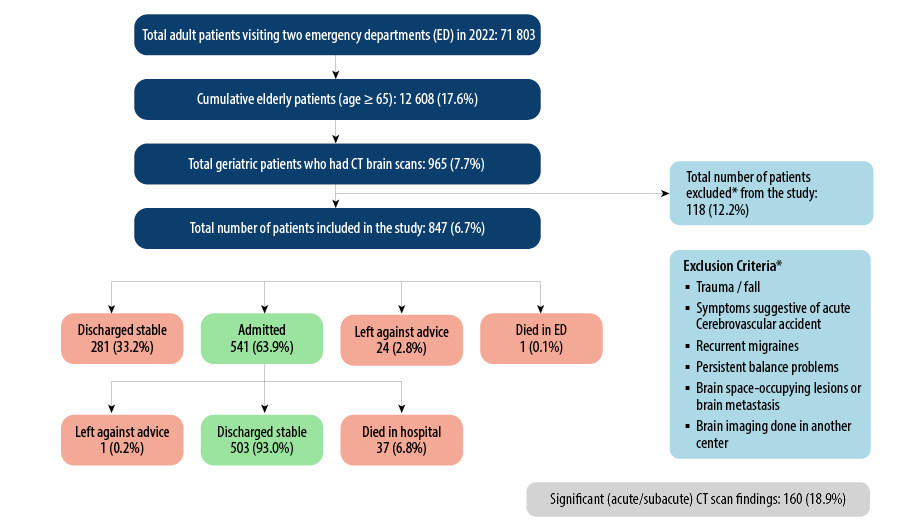 Figure 1: Strengthening the reporting of observational studies in epidemiology (STROBE) statement.
Figure 1: Strengthening the reporting of observational studies in epidemiology (STROBE) statement.
Table 1: Demographics and medical history of the study population, n = 847.
|
Mean age, mean ± SD, years
|
76.0 ± 7.7
|
|
Sex, male
|
402 (47.5)
|
|
Comorbidities
|
|
Hypertension
|
605 (71.4)
|
|
Diabetes mellitus
|
445 (52.5)
|
|
Dyslipidemia
|
325 (38.4)
|
|
Ischemic heart disease
|
297 (35.1)
|
|
Chronic kidney disease
|
172 (20.3)
|
|
Cerebrovascular accident
|
65 (7.7)
|
|
Neurological disorders*
|
139 (16.4)
|
|
Reactive airway disease/smoker
|
71 (8.4)
|
|
Chronic liver disease
|
36 (4.3)
|
|
Solid or hematological malignancies
|
126 (14.9)
|
|
Others#
|
106 (12.5)
|
|
Use of antiplatelet(s) drugs
|
375 (44.3)
|
*including seizure disorder, Alzheimer’s disease, Parkinson’s disease, and dementia. #Arrhythmias, heart failure, valvular heart disease, obstructive sleep apnea, hypothyroidism, gastric reflux disease, osteoarthritis, benign prostatic hyperplasia, hypovitaminosis, anemia.
Table 2: Patient complaints and abnormal vital signs on emergency department (ED) presentation, n = 847.
|
Presenting complaints to the ED
|
|
Fever
|
483 (57.0)
|
|
Drowsiness
|
397 (46.9)
|
|
Dizziness
|
254 (30.0)
|
|
Headache
|
203 (24.0)
|
|
Nausea/vomiting/abdominal pain
|
201 (23.7)
|
|
Transient loss of consciousness
|
181 (21.4)
|
|
Fatigue
|
172 (20.3)
|
|
Vertigo
|
97 (11.5)
|
|
Others*
|
178 (21.0)
|
|
Abnormal vital signs at presentation
|
|
Tachycardia (heart rate ≥ 100/min)
|
179 (21.1)
|
|
Bradycardia (heart rate ≤ 60/min)
|
63 (7.4)
|
|
Tachypnea (respiratory rate > 20/ min)
|
229 (27.0)
|
|
Systolic blood pressure (≤ 90 mmHg)
|
38 (4.5)
|
|
Systolic blood pressure (≥ 180 mmHg)
|
94 (11.1)
|
|
SPO2 (≤ 94% in room air)
|
163 (19.2)
|
|
Random blood sugar level (< 3.0 mmol/L)
|
18 (2.1)
|
|
Normal sensorium
|
602 (71.1)
|
|
Glasgow coma scale
|
|
|
15–13
|
165 (19.5)
|
|
12–8
|
57 (6.7)
|
All these patients presented to the ED with generalized weakness, along with the aforementioned symptoms. Others*: Breathing difficulty, decreased or no urine output, chest pain, limb pain and swelling, etc. *Patients on anticoagulation and those with trauma were excluded. Additionally, based on the study (Blunt Head Injury in the Elderly: Analysis of the NEXUS II Injury Cohort), patients who sustained accidental falls at ground level were also excluded.
Table 3: Tabulation of abnormal radiological, laboratory, and electrocardiogram (ECG) findings, n = 847.
|
Abnormal CT scan findings of the brain
|
|
Age-related brain atrophy and/or chronic microvascular ischemic changes
|
385 (45.5)
|
|
Acute ischemic changes
|
28 (3.3)
|
|
Subacute ischemic changes
|
66 (7.8)
|
|
Acute on chronic subdural hematoma/intracranial hematoma
|
29 (3.4)
|
|
New space-occupying lesion/abscess
|
37 (4.4)
|
|
Others*
|
52 (6.1)
|
|
Abnormal chest radiograph findings1
|
|
|
Infiltrates, new consolidation
|
79 (18.9)^
|
|
Interstitial edema other features of heart failure
|
112 (26.7)^
|
|
Features of infected heart failure
|
72 (17.2)^
|
|
Abnormal hematological and biochemical markers, urine dipstick results, and ECG findings
|
|
Hypernatremia (> 145 mmol/L)
|
33 (3.9)
|
|
Mild hyponatremia (serum sodium:
130–135 mmol/L)
|
211 (24.9)
|
|
moderate hyponatremia (serum sodium: 125–129 mmol/L)
|
100 (11.8)
|
|
profound hyponatremia (serum sodium:
< 125 mmol/L)
|
63 (7.4)
|
|
.subgroup A (121–125 mmol/L)
|
30 (3.5)
|
|
.subgroup B (116–120 mmol/L)
|
21 (2.5)
|
|
.subgroup C (≤ 115 mmol/L)
|
12 (1.4)
|
|
Hyperkalemia (> 5.5 mmol/L)
|
55 (6.5)
|
|
Mild hypokalemia (3–3.4 mmol/L)
|
35 (4.1)
|
|
Moderate hypokalemia (2.5–3 mmol/L)
|
9 (1.1)
|
|
Severe hypokalemia (< 2.5 mmol/L)
|
1 (0.1)
|
|
High creatinine (59–104 mmol/L)
|
343 (40.5)
|
|
High urea (2.8–8.1mmol/L)
|
371 (43.8)
|
|
Abnormal white cell count (2.2–10.0 109/L)
|
218 (25.7)
|
|
Abnormal platelet count (150–450 109/L)
|
85 (10.0)
|
|
C-reactive protein (0–5 mg/L)
|
629 (74.3)
|
|
Raised alanine aminotransferase (ALT) test (> 41 U/L)
|
105 (12.4)
|
|
Raised total bilirubin (> 17 mmol/L)
|
132 (15.6)
|
|
Abnormal albumin (32–45 g/L)
|
77 (9.1)
|
|
Abnormal coagulation profile
|
92 (22.3)#
|
|
Urine dipstick (leukocytes/nitrates/blood)
|
152 (23.9)$
|
CT: computed tomography. *Mastoiditis, normal pressure hydrocephalus, pan-sinusitis. A chest radiograph was performed on 419^ patients with suspected respiratory infections, a coagulation profile was conducted for 412# patients, urine dipsticks were used for 636$ patients with suspected urinary sepsis, and a 12-lead ECG was done for 518! patients. These investigations were not performed on all patients, which is one of the limitation of this retrospective study. The percentages were calculated based on the number of patients who underwent these specific tests, while the remaining investigations were part of the routine blood workup.
For acute and/or acute-on-chronic subdural hematoma [Figure 2a], the area under the curve (AUC) was 0.41, indicating performance worse than random guessing (AUC of 0.5). The optimal threshold of 0.122 maximized the difference between the true positive and false positive rates. Similarly, for acute and/or subacute ischemic cerebrovascular accident [Figure 2b], the AUC was 0.47, also indicating below-average performance compared to random guessing. The optimal threshold of 0.188 achieved the highest difference between the true positive and false positive rates. For significant new and/or acute CT findings [Figure 3a], the AUC was 0.45, suggesting performance worse than random guessing. The optimal threshold of 0.026 maximized the true positive and false positive rate difference. Likewise, for newly detected SOLs [Figure 3b], the AUC was 0.43, indicating performance below random guessing. The optimal threshold of 0.144 maximized the difference between the true positive and false positive rates, as determined by Youden's J statistic.
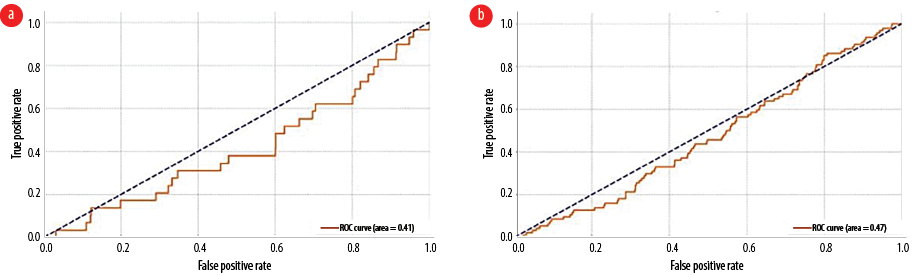 Figure 2: Receiver operating characteristic curve and area under the curve analysis of CT scan in detecting (a) acute/acute-on-chronic subdural hematoma and (b) acute/subacute ischemic cerebrovascular accident.
Figure 2: Receiver operating characteristic curve and area under the curve analysis of CT scan in detecting (a) acute/acute-on-chronic subdural hematoma and (b) acute/subacute ischemic cerebrovascular accident.
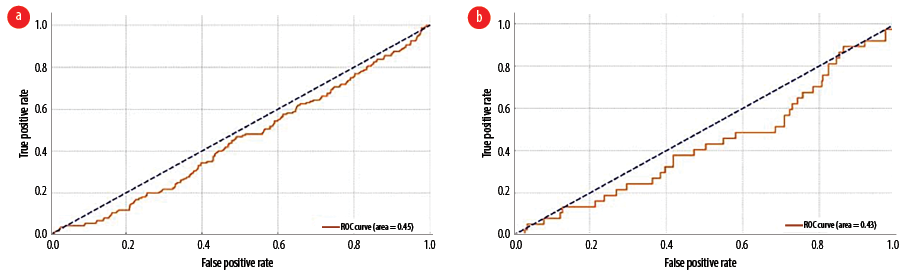 Figure 3: Receiver operating characteristic curve and area under the curve analysis of CT scan in detecting (a) significant new and/or acute CT findings and (b) newly detected space-occupying lesions.
Figure 3: Receiver operating characteristic curve and area under the curve analysis of CT scan in detecting (a) significant new and/or acute CT findings and (b) newly detected space-occupying lesions.
For acute and/or acute-on-chronic subdural hematoma, the AUC was 0.41, indicating performance worse than random guessing (AUC of 0.5). The optimal threshold of 0.122 maximizes the difference between the true positive rate and false positive rate (Youden's J statistic). Similarly, for acute and/or subacute ischemic cerebrovascular accident, the AUC was 0.47, also indicating performance worse than random guessing (AUC of 0.5). The optimal threshold of 0.188 maximizes the difference between the true positive rate and false positive rate (Youden's J statistic).
For significant new and/or acute CT findings, the AUC was 0.45, indicating performance worse than random guessing (AUC of 0.5). The optimal threshold, 0.026, maximizes the difference between the true positive and false positive rates (Youden's J statistic). Similarly, for newly detected SOLs, the AUC was 0.43, also indicating below-average performance compared to random guessing (AUC of 0.5). The optimal threshold of 0.144 maximizes the true positive and false positive rate difference (Youden's J statistic).
Detailed ED and hospital disposition data are provided in Figure 1, offering insights into patient management outcomes.
Discussion
A common challenge in busy EDs is diagnosing geriatric patients who present with generalized weakness. Approximately 20% of geriatric patients arrive at the ED with such nonspecific complaints, which require thorough evaluation due to their association with adverse outcomes.2,10 The widespread use of CT scans has transformed brain disease evaluation, driven by factors like easy access, high image quality, non-invasiveness, minimal time consumption, patient pressure, and fear of litigation.11–13 However, the increased use of head CTs for non-trauma patients has raised public health concerns, including higher costs and resource wastage.
Our study focused on patients presenting with generalized weakness who underwent head CT imaging, revealing a low incidence of acute intracranial abnormalities. The diagnostic performance of head CTs in detecting acute cerebrovascular accidents and other significant findings was limited, as reflected in suboptimal ROC analysis thresholds. Smith-Bindman et al,14 reported similar limitations in the diagnostic accuracy of CT scans for non-traumatic presentations in the elderly. Comparatively, Grossman et al,15 found that head CTs were more likely to detect abnormalities with focal neurological deficits or a history of consciousness loss, we observed that generalized weakness alone was not a strong predictor of acute findings. Our findings suggest the need for more selective use of head CTs to avoid unnecessary costs and resource utilization. We also included patients who presented with generalized weakness and other symptoms, and who experienced cardiac arrest during their ED stay. CT scans performed as part of post-cardiac arrest care were included, some of which revealed acute findings. Excluding these cases would have halved the number of acute findings; however, given the small sample size of such patients, the exclusion would not have significantly impacted the statistical analysis. Other research has shown that factors such as nausea and/or vomiting, history of malignancy, coagulation disorders, and comorbidities like hypertension also influence abnormal head CT findings.16–19 Minor predictors of abnormal head CT findings include headache and dizziness.20 Research shows that only a small percentage of head CT examinations in non-trauma patients reveal abnormalities. Other studies indicate that head CTs are more likely to detect significant findings in patients with specific risk factors such as advanced age, focal neurological deficits, history of trauma, or a history of malignancy.21,22
Based on the literature and our study findings, we recommend obtaining a complete blood count, a basic metabolic panel, an electrocardiogram, and chest radiograph. Additional tests might include erythrocyte sedimentation rate/C-reactive protein for inflammatory processes, thyroid-stimulating hormone for hypothyroidism, and urinalysis assess the prevalence of asymptomatic bacteriuria.23 A 12-lead electrocardiogram is also advised, as myocardial infarction can present as generalized weakness in this population.24 Even if a thorough review of the patient's complete history, including input from relatives, clinical examination findings, and laboratory investigations, fails to yield a definitive diagnosis, brain imaging may be warranted. Despite the low diagnostic yield, a negative head CT scan can reassure patients, facilitate faster discharge, and reduce unnecessary observation periods, thus shortening the length of stay. Overall, our findings support a more selective approach to head CT imaging in geriatric patients with generalized weakness, emphasizing clinical evaluation and judicious use of imaging based on specific clinical indicators. This strategy optimizes resource use, minimizes unnecessary radiation exposure, and contributes to better patient outcomes and more efficient healthcare delivery.
The study's robustness is evident in its calculation of a sample size with 1.2% precision, ensuring reliability with a larger cohort involving two major national centers in a retrospective design that minimizes exposure risks. Using ROC curve analysis, it demonstrates AUC metrics and graph plotting to determine optimal thresholds. Notably, it features one of the largest patient cohorts, enriching the database and enhancing overall significance. However, the retrospective nature of this study meant that charts with missing data were excluded, which could affect the dataset's completeness. While medical chart documentation was generally accurate, the consistency across different variables varied. Additionally, the decision to perform a CT scan depended on the treating physicians, whose preferences and practices may have introduced variability.
Conclusion
Our study demonstrates that CT brain scans offer limited diagnostic value for detecting acute intracranial pathology in elderly patients with generalized weakness, as confirmed by ROC curve analysis. The statistical results revealed suboptimal performance, with AUCs of 0.41 for acute/acute-on-chronic subdural hematomas, 0.47 for ischemic cerebrovascular accidents, 0.45 for significant new CT findings, and 0.43 for SOLs—all suggesting performance worse than random guessing. These findings underscore the limited utility of CT scans in this cohort. Furthermore, the study highlights the diverse clinical presentations of geriatric patients and emphasizes the need for tailored management approaches. This aligns with the existing literature, which advocates a broad differential diagnosis in geriatric care, where patient characteristics may not always direct diagnostic decision-making.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
Acknowledgments
We would like to thank Dr. Ali Ahmed Al Subhi, Dr. Fatma Mohammed Hassan AlShehhi, and Dr. Sara Mohammed Alhabsi for their contributions to data collection.
references
- 1. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 2019 Jan;99(1):427-511.
- 2. Bhalla MC, Wilber ST, Stiffler KA, Ondrejka JE, Gerson LW. Weakness and fatigue in older ED patients in the United States. Am J Emerg Med 2014 Nov;32(11):1395-1398.
- 3. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007 May;55(5):780-791.
- 4. Morley JE. The importance of geriatric syndromes. Mo Med 2017;114(2):99-100.
- 5. Norman K, Haß U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients 2021 Aug;13(8):2764.
- 6. Chew WM, Birnbaumer DM. Evaluation of the elderly patient with weakness: an evidence based approach. Emerg Med Clin North Am 1999 Feb;17(1):265-278.
- 7. Day GS, Long A, Morris JC. Assessing the reliability of reported medical history in older adults. J Alzheimers Dis 2020;78(2):643-652.
- 8. Segard J, Montassier E, Trewick D, Le Conte P, Guillon B, Berrut G. Urgent computed tomography brain scan for elderly patients: can we improve its diagnostic yield? Eur J Emerg Med 2013 Feb;20(1):51-53.
- 9. Bennett J, Brown C, Brewer K, Johnson R. 273: use of head computed tomography in elderly patients presenting after a fall: prevalence and predictors of intracranial injury. Ann Emerg Med 2010 Sep;56(3):S90.
- 10. Calhoun EA, Shih RD, Hughes PG, Solano JJ, Clayton LM, Alter SM. Head computerized tomography in emergency department evaluation of the geriatric patient with generalized weakness. J Am Coll Emerg Physicians Open 2023 Jun;4(4):e12998.
- 11. Bhargava R. CT imaging in neurocritical care. Indian J Crit Care Med 2019 Jun;23(S2)(Suppl 2):S98-S103.
- 12. Acharya R, Kafle S, Shrestha DB, Sedhai YR, Ghimire M, Khanal K, et al. Use of computed tomography of the head in patients with acute atraumatic altered mental status: a systematic review and meta-analysis. JAMA Netw Open 2022 Nov;5(11):e2242805.
- 13. Patel PR, De Jesus OC. Scan. 2023 Jan 2. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
- 14. Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA 2012 Jun;307(22):2400-2409.
- 15. Grossman SA, Fischer C, Bar JL, Lipsitz LA, Mottley L, Sands K, et al. The yield of head CT in syncope: a pilot study. Intern Emerg Med 2007 Mar;2(1):46-49.
- 16. Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G, et al. Cancer-associated stroke: pathophysiology, detection and management (review). Int J Oncol 2019 Mar;54(3):779-796.
- 17. Alawneh KZ, Raffee LA, Oqlat AA, Oglat AA, Al Qawasmeh M, Ali MK, et al. The utility of brain CT scan modality in the management of dizziness at the emergency department: a retrospective single-center study. Ann Med Surg (Lond) 2021 Mar;64:102220.
- 18. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res 2017 Feb;120(3):472-495.
- 19. Lawhn-Heath C, Buckle C, Christoforidis G, Straus C. Utility of head CT in the evaluation of vertigo/dizziness in the emergency department. Emerg Radiol 2013 Jan;20(1):45-49.
- 20. Filler L, Akhter M, Nimlos P. Evaluation and management of the emergency department headache. Semin Neurol 2019 Feb;39(1):20-26.
- 21. Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000 Jul;343(2):100-105.
- 22. Mower WR, Hoffman JR, Herbert M, Wolfson AB, Pollack CV Jr, Zucker MI; NEXUS II Investigators. Developing a decision instrument to guide computed tomographic imaging of blunt head injury patients. J Trauma 2005 Oct;59(4):954-959.
- 23. Schippinger W. Comprehensive geriatric assessment. Wien Med Wochenschr 2022 Apr;172(5-6):122-125.
- 24. Gupta M, Tabas JA, Kohn MA. Presenting complaint among patients with myocardial infarction who present to an urban, public hospital emergency department. Ann Emerg Med 2002 Aug;40(2):180-186.