Aspirin-exacerbated respiratory disease (AERD) or nonsteroidal anti-inflammatory drug (NSAID)-ERD (NERD) is defined as hypersensitivity to aspirin or NSAIDs that results in a chronic eosinophilic, inflammatory disorder of the respiratory tract in patients with asthma and chronic rhinosinusitis with nasal polyps.1 Aspirin and NSAIDs have been used in many diseases, such as respiratory, rheumatology, and cardiovascular diseases.2–5 Regular aspirin use was linked to a lower rate of chronic obstructive pulmonary disease (COPD) flare-ups, decreased shortness of breath, and improved quality of life.2 However, unlike most patients with identical clinical features, patients with NERD have respiratory reactions after ingesting aspirin and other NSAIDs.3 These allergic reactions usually involve the upper respiratory system (nasal stuffiness, runny nose, and sneezing) and lower respiratory system (throat spasms, cough, and wheezing). Gastrointestinal symptoms (stomach pain and nausea) and skin symptoms (redness and hives) are less common but almost always occur along with some respiratory symptoms.6
NERD is acquired from late childhood to adulthood; the median age at onset is approximately 30 years.7 There are no accurate data on the prevalence of NERD in the general population or among patients with asthma, nasal polyposis, or both.6 A European multi-center study found the prevalence of respiratory allergic reactions to NSAIDs to be 1.9%. In Poland, the prevalence was 0.6%; in Finland, 1.2%; and in Sweden, it was 1.3%.8 It has been hypothesized that in hypersensitive patients, inhibition of the prostaglandin (PG) E2 pathway by NSAIDs triggers the activation of inflammatory cells such as mast cells, basophils, eosinophils, and potentially platelets. This leads to the release of substances like cysteinyl leukotrienes, PGD2, histamine, and tryptase, which then cause the development of clinical symptoms. However, the exact mechanism behind this specific activation of inflammatory cells is still debated. Possibilities include increased susceptibility of cyclooxygenase-1 to inhibition by NSAIDs, an inherent deficiency in PGE2 production by cyclooxygenase-2, and/or abnormal function of PGE2 receptors in patients with NERD.1 Furthermore, there is still uncertainty regarding the risk factors for NERD. This meta-analysis summarizes studies that have investigated the potential risk factors for NERD.
Methods
Following the reporting guidelines recommended in Preferred Reporting Items for Systematic Reviews and Meta-Analyses,9 we systematically reviewed of the scientific literature to identify studies that investigated factors associated with NERD.
Two authors (ENN and AABW) search the electronic databases MEDLINE and Scopus. The search was conducted without date and language limitations. The search algorithm applied in the free text was: NSAID-exacerbated respiratory disease OR aspirin-exacerbated respiratory disease OR aspirin-sensitive asthma OR aspirin-induced asthma OR Samter’s triad OR aspirin triad OR Widal’s triad AND (risk OR ratio.
Titles and abstracts of articles identified with the initial search were screened, and articles were excluded from further analysis based on title (not relevant) or abstract (unequivocal presence of excluding features). Excluding features were study designs other than cohort, case-control, or cross-sectional designs. This review was used as a reference only. Risk factors were initially grouped for those in the general population and those in specific populations and then grouped into the following domains: age, gender, body mass index, family history, smoking, and atopy.
A literature search identified 161 articles from MEDLINE and SCOPUS. Nineteen studies met the eligibility criteria and remained for qualitative synthesis [Figure 1].
 Figure 1: Preferred Reporting Items from Systematic Reviews and Meta-Analyses demonstrates that the systematic review excluded and examined the literature.
Figure 1: Preferred Reporting Items from Systematic Reviews and Meta-Analyses demonstrates that the systematic review excluded and examined the literature.
Evaluation of the quality of reports on the studies
Two reviewers (D and EI) independently rated the title, abstract, methods, results, discussion, and other parts of each study according to the standard items listed in the Joanna Briggs Institute Qualitative Assessment and Review Instrument.10 The total score range was zero to eight, with each item accounting for one point. All discrepancies were resolved by group discussion and consensus. The inter-rater reliability of the two authors for this quality measure was strong, with an intraclass correlation coefficient of 0.74 (95% CI: 0.15–0.94) [Table 1].
Table 1: Evaluation of the qualifying studies using the Joanna Briggs Institute guidelines.
|
Heidi, Pinja8
|
Y
|
Y
|
Y
|
Y
|
Y
|
N
|
Y
|
Y
|
|
Eriksson, Ekerljung9
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
|
Bavbek, Yılmaz10
|
Y
|
Y
|
N
|
Y
|
Y
|
Y
|
Y
|
Y
|
|
Helevä, Murtomäki11
|
Y
|
Y
|
N
|
Y
|
Y
|
Y
|
Y
|
Y
|
|
Nabavi, Esmaeilzadeh12
|
Y
|
Y
|
Y
|
Y
|
Y
|
N
|
Y
|
Y
|
|
Chang, Park13
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
|
Lee, Bae14
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Y
|
Q1: Were the criteria for inclusion in the sample clearly defined? Q2: Were the study subjects and the setting described in detail? Q3: Was the exposure measured in a valid and reliable way? Q4: Were objective, standard criteria used for the measurement of the condition? Q5: Were confounding factors identified? Q6: Were strategies to deal with confounding factors stated? Q7: Were the outcomes measured in a valid and reliable way? Q8: Was appropriate statistical analysis used? Y: Yes; N: No. Citations in this table corresponds with the citations in the result section.
Statistical analysis
Descriptive statistics, including mean, SD, frequency, and percentage, were used to describe the basic information of each study in the systematic review. Meta-analysis and forest plots were used to integrate all reviewed studies and estimate the pooled odds ratio (OR) and 95% CI of risk factors associated with NERD compared with non-NERD groups for each reviewed study.11 According to DerSimonian and Laird,12 I2 and Cohran’s Q statistics assessed homogeneity across studies. A random-effects model was used to calculate the pooled OR if the I2 > 50% and the p < 0.10 on Q statistic were shown as heterogeneity of the reviewed articles.12 In addition, because both outcomes are continuous data, Bigg’s test, if the p-value is < 10%, and funnel plot was used to remove articles with publication bias that might influence the analysis results.11 The MedCalc 12 statistical software performed all analyses, and the significance was 5%.
Results
The characteristics and results of the observational studies are summarized in Table 2 for the risk factors of NERD. All included studies had a non-experimental design and were published between 2003 and 2022 [Table 2].
Table 2: Summary of studies investigating the risk factors for nonsteroidal anti-inflammatory drug-exacerbated respiratory disease.
|
Heidi, Pinja8
|
Finland
|
2016
|
16 000
|
General population
|
Cross-sectional
Prevalence of NERD 1,4%
|
Age 40–59 years old
Age 60–69 years old
Family history of asthma
Family history of allergic rhinitis
Ever smoking
Female
BMI ≥ 30 kg/m2
|
2.11 (1.20–3.76)
3.08 (1.70–5.64)
2.22 (1.52–3.25)
2.47 (1.60–3.83)
1.34 (0.92–1.97)
1.49 (1.00–2.22)
1.14 (0.67–1.95)
|
|
Eriksson, Ekerljung9
|
Sweden
|
2008
|
18 087
|
General population
|
Cross-sectional
Prevalence of NERD 0.5%
|
BMI ≥ 35
BMI 30–35
Family history of asthma
Female
Exposure:
Airborne occupational
Visible mold at home
Ever smoking
Age 36–55 years old
Age 56–75 years old
Airborne occupational exposure
Visible mould at home
Water damage at home
Farm childhood
|
12.07 (2.49–58.50)
7.31 (1.63–32.8)
2.36 (1.51–3.67)
1.96 (1.23–3.13)
2.77 (1.72–4.48)
2.63 (1.45–4.74)
2.99 (1.92–4.66)
1.13 (0.65–1.94)
0.70 (0.36–1.35)
2.77 (1.72–4.48)
2.63 (1.45–4.74)
1.54 (0.87–2.73)
1.10 (0.59–2.08)
|
|
Bavbek, Yılmaz10
|
Turkey
|
2007
|
1344
|
Adult with asthma
|
Cross-sectional
Prevalence of NERD 13.39%
|
Atopy
Female
Ever smoking
Family history of asthma
|
0.81 (0.59–1.11)
1.12 (0.74–1.67)
0.67 (0.35–1.27)
4.90 (2.56–9.37)
|
|
Helevä, Murtomäki11
|
Finland
|
1996–1997
|
1350
|
Adult-onset asthma
|
Cross-sectional
Prevalence of NERD 11.3%
|
Female
BMI < 20
BMI > 30
Atopy
Osteoarthritis
Rheumatoid arthritis
Environmental tobacco smoke exposure
Four or later child
Ever smoking
|
3.03 (1.98–4.64)
1.15 (0.54–2.47)
0.60 (0.43–0.85)
3.06 (2.09–4.50)
1.90 (1.23–2.95)
2.04 (1.06–3.93)
2.05 (1.09–3.85)
1.63 (1.10–2.41)
0.70 (0.50–0.98)
|
|
Nabavi, Esmaeilzadeh12
|
Iran
|
2012
|
80
|
Chronic rhinosinusitis and osis
|
Case-control
|
Female
History of ASA hypersensitivity
Blood esosinophil > 450
Atopy
Nasal smear eosinophilia >10%
|
2.28 (0.93–5.61)
10.92 (2.28–52.20)
1.06 (0.28–3.98)
1.12 (0.46–2.70)
1.06 (0.28–3.98)
|
|
Chang, Ding13
|
California
|
2003–2009
|
260
|
General population
|
Case-control
|
Age > 40 years old
Female
ETS exposure
|
0.86 (0.57–1.29)
1.80 (1.27–2.55)
2.98 (1.91–4.63)
|
|
Pasaje, Bae14
|
South Korea
|
2003–2008
|
592
|
Asthmatic patients
Mean age: 46.15 years
|
Case-control
|
Female
C6 polymorphism
Ever smoking
Atopy
|
0.93 (0.66–1.32)
Not significant
0.64 (0.42–0.98)
0.82 (0.57–1.17)
|
|
Lee, Bae15
|
South
Korea
|
Published: 2012
|
592
|
Asthmatic patients
Mean age: 46.15 years
|
Case-control
|
Female
CD55 polymorphism
Ever smoking
Atopy
|
0.93 (0.66–1.32)
Not significant
0.64 (0.41–0.97)
0.82 (0.57–1.17)
|
|
Chang, Park16
|
Korea
|
2014
|
1019
|
Asthmatic patients
|
Case-control
|
Female
Ever smoking
FABP1 polymorphism
|
0.89 (0.65–1.20)
0.55 (0.38–0.80)
Not Significant
|
|
Lee, Bae17
|
Korea
|
2012
|
189
|
Asthmatic patients
|
Case-control
|
Atopy
Ever smoking
Female
TRIM 26 polymorphism
|
1.18 (0.66–2.11)
1.33 (0.67–2.65)
0.64 (0.34–1.19)
Not significant
|
|
Pasaje, Bae18
|
Korea
|
2003–2008
|
592
|
Asthmatic patients
|
Case-control
|
Atopy
Female
K1F1 polymorphism
|
1.18 (0.66–2.11)
0.64 (0.34–1.19)
Not significant
|
|
Park, Kim19
|
Korea
|
2012
|
1940
|
Asthmatic patients
|
Case-control
|
Female
Ever smoking
Atopy
HLA-DPB1 gene polymorphism
|
1.01 (0.70–1.44)
0.59 (0.38–0.91)
1.03 (0.72–1.46)
2.40 (1.68–3.42)
|
|
Karakaya, Demir20
|
Turkiye
|
1999
|
344
|
General population
|
Case-control
|
Ever smoking
Female
Family history of asthma
Atopy
|
0.76 (0.47–1.22)
0.81 (0.50–1.34)
0.68 (0.32–1.43)
2.17 (1.28–3.66)
|
|
Rebelo Gomes, Geraldes21
|
Portugal
|
2013
|
227
|
Atopic patients
|
Case-control
|
Female
Immediate time to the reaction
atopy
|
0.79 (0.36–1.75)
11.94 (4.68–30.50)
0.82 (0.37–1.86)
|
|
Karakaya, Celebioglu22
|
Turkiye
|
1991–2010
|
1137
|
Atopic patients
|
Cross-sectional
|
Female
Ever smoking
Immediate reaction to NSAID
Family history of asthma
Atopy
|
1.34 (1.02–1.77)
0.64 (0.49–0.84)
1.76 (1.31–2.35)
0.54 (0.33–0.89)
1.49 (1.10–2.02)
|
|
Makowska, Burney6
|
22 centers across Europe
|
2008–2009
|
62 737
|
General population
|
Cross-sectional
|
Age > 45 years old
Ever smoking
Female
|
1.44 (1.28–1.62)
1.64 (1.44–1.86)
1.74 (1.54–1.96)
|
|
Jang, Park23
|
South Korea
|
2006
|
667
|
Asthmatic patients
|
Cross-sectional
|
Female
Ever smoking
Atopy
BMI > 30
|
0.99 (0.67–1.46)
0.72 (0.46–1.12)
1.62 (1.11–2.35)
0.85 (0.40–1.81)
|
|
Shin, Park24
|
South Korea
|
2014
|
560
|
Asthmatic patients
|
|
Female
Ever smoking
Atopy
|
0.95 (0.67–1.33)
0.57 (0.37–0.87)
1.70 (1.21–2.38)
|
BMI: body mass index; OR: odds ratio; ETS: environmental tobacco smoke.; cumulative exposure was classified from zero to three exposures, calculating smoking (current or ex-smoking), secondhand smoke (smoke exposure at home or work), and occupational exposure to vapors, gases, dust, and fumes. Citations in this table corresponds with the citations in the result section.
A total of 161 papers were initially retrieved through database searching. After screening titles and abstracts, 54 articles remained to be reviewed for eligibility. Among them, 31 were excluded after full-text screening. Of the 23 eligible studies, we excluded four articles. Our final total was 19 eligible studies. An overview of the study selection process is presented in Figure 1.
Association of gender on NERD
The meta-analysis [Figure 2a] displays the pooled OR on gender associated with NERD in 17 studies1–7,9,10,12–19 compared to the non-NERD group. The pooled data included 95 007 samples (NERD = 3607 vs. non-NERD = 91 400), and its heterogeneity was high (I2 = 77.38%) and significant (Q = 70.73; p < 0.001). Therefore, the random-effects model was used, showing that females had 1.22 times (OR = 1.22; p = 0.048) significantly higher than males with NERD than the non-NERD group. Publication bias was noted from the funnel plot (Figure 2b). Sensitivity analysis was performed and suggested that several studies4,6,9,10,13,16 should be excluded, and its publication bias was changed to low (Egger’s test, p = 0.692), and the heterogeneity test was found significant (Q = 15.51; p = 0.115) (Figure 2c). Therefore, a fixed-effects model (I2 = 35.53%) was used for 11 studies, and it showed that females had 1.16 times (OR = 1.16, 95% CI: 1.02–1.31; p = 0.023) significantly higher than males with NERD than the non-NERD group.
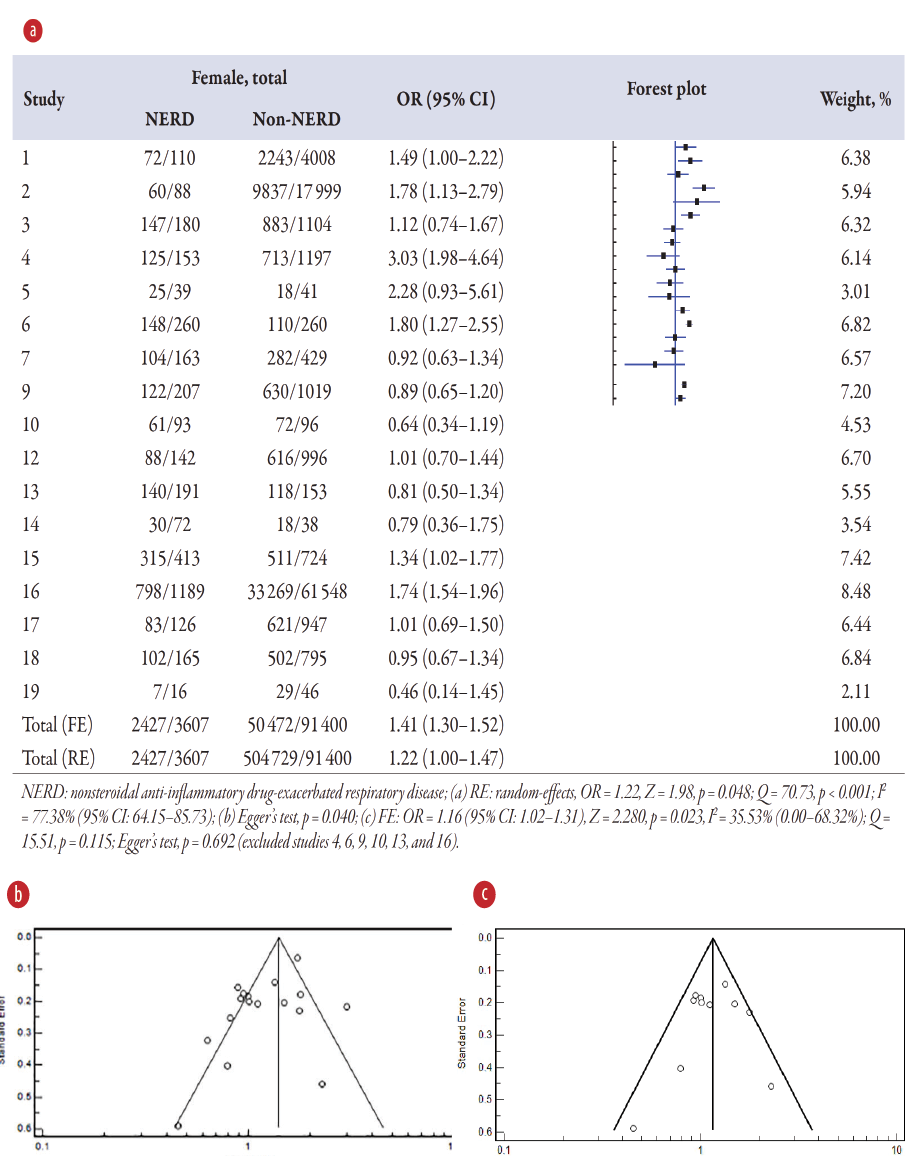 Figure 2: (a) Overview of the meta-analysis results of gender on NERD and non-NERD groups. (b) Funnel plot for 17 studies. (c) Funnel plot for 11 studies after excluding six studies with publication bias.
Figure 2: (a) Overview of the meta-analysis results of gender on NERD and non-NERD groups. (b) Funnel plot for 17 studies. (c) Funnel plot for 11 studies after excluding six studies with publication bias.
Association of atopy on NERD
The meta-analysis [Figure 3a] displays the pooled OR on atopy associated with NERD in 11 studies3–5,7,10,12–15,17,18 compared to the non-NERD group. The pooled data included 8258 samples (NERD = 1713 vs. non-NERD = 6545), and its heterogeneity was high (I2 = 77.96%) and significant (Q = 45.36; p < 0.001). Therefore, the random-effects model was used, showing that atopy is 1.34 times (OR = 1.34; p = 0.035) significantly higher than without atopy on NERD than the non-NERD group. Publication bias was noted from the funnel plot [Figure 3b]. Sensitivity analysis was performed and suggested that three studies3,4,7 should be excluded, and its publication bias was changed to low (Egger’s test, p = 0.472), and the heterogeneity test was found not significant (Q = 9.82; p = 0.199) [Figure 3c]. Therefore, a fixed-effects model (I2 = 28.74%) was used for eight studies, and showed that atopy had 1.43 times (OR = 1.43, 95% CI: 1.23–1.66; p < 0.001) significantly higher than non-atopy on NERD than the non-NERD group.
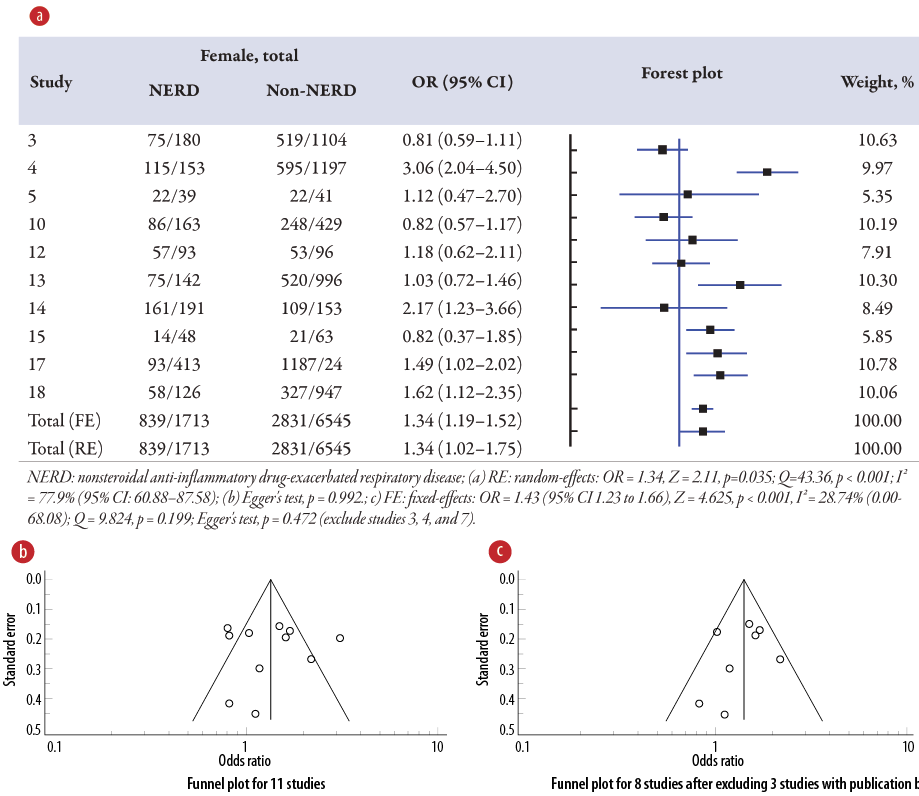 Figure 3: (a) Overview of the meta-analysis results of atopy on NERD and non-NERD groups. (b) Funnel plot for 11 studies. (c) Funnel plot for eight studies after excluding three studies with
Figure 3: (a) Overview of the meta-analysis results of atopy on NERD and non-NERD groups. (b) Funnel plot for 11 studies. (c) Funnel plot for eight studies after excluding three studies with
publication bias.
Association of smoking on NERD
The meta-analysis [Figure 4a] displays the pooled OR of smoking associated with NERD in 15 studies, including two studies of environmental tobacco smoke1–4,6,7,9,10,12,13,15–18 compared to the non-NERD group. The pooled data included 95 953 samples (NERD = 3548 vs. non-NERD = 92 405), and its heterogeneity was high (I2 = 90.58%) and significant (Q = 148.67; p < 0.001). Therefore, the random-effects model was used, showing that smoking has no (OR = 1.00; p = 0.971) significant association with NERD compared to the non-NERD group. Publication bias was noted from the funnel plot [Figure 4b]. Sensitivity analysis was performed and suggested that 11 studies2–4,7,9,12,13,15,17,18 should be excluded, and its publication bias was changed to low (Egger’s test, p = 0.715), and the heterogeneity test was found not significant (Q = 1.78; p = 0.619) [Figure 4c]. Therefore, a fixed-effects model (I2 = 1.78%) was used for four studies, and it showed that smoking was 1.60 times (OR = 1.60, 95% CI: 1.43–1.80; p < 0.001) significantly higher than those without smoking on NERD than the non-NERD group.
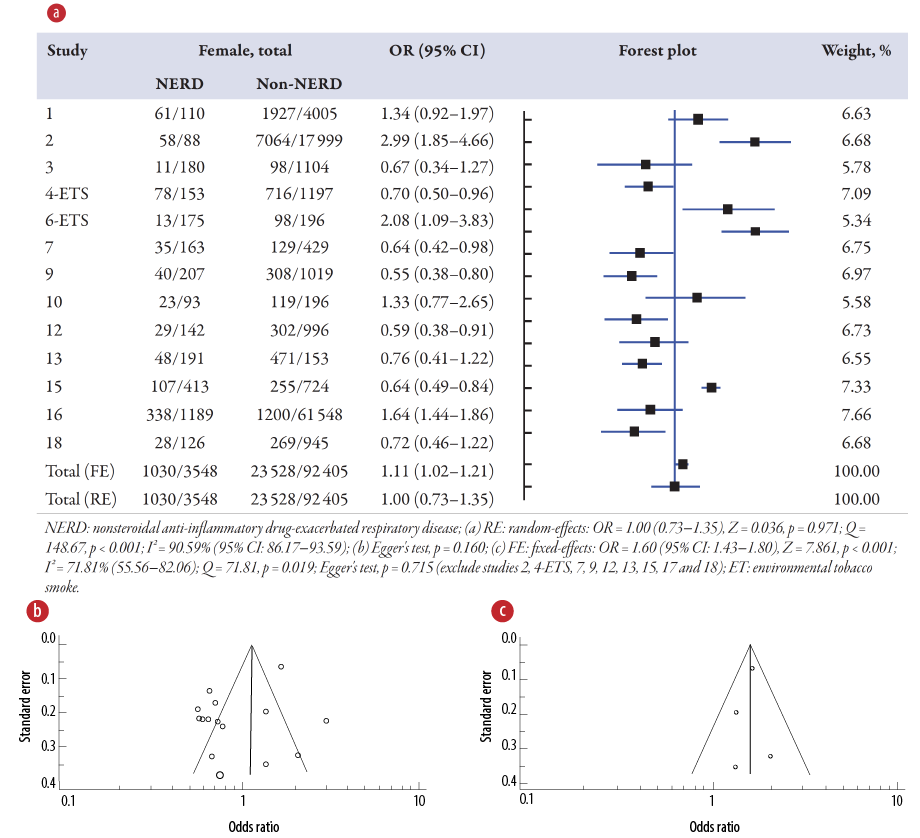 Figure 4: (a) Overview of the meta-analysis results of smoking in NERD and non-NERD groups. (b) Funnel plot for 15 studies. (c) Funnel plot for four studies after excluding 11 studies with publication bias.
Figure 4: (a) Overview of the meta-analysis results of smoking in NERD and non-NERD groups. (b) Funnel plot for 15 studies. (c) Funnel plot for four studies after excluding 11 studies with publication bias.
Association of asthma on NERD
The meta-analysis [Figure 5a] displays the pooled OR of asthma associated with NERD in five studies1–3,13,15 compared to the non-NERD group. The pooled data included 24 970 samples (NERD = 982 vs. non-NERD = 23 988), and its heterogeneity was high (I2 = 90.25%) and significant (Q = 41.04; p < 0.001). Therefore, the random-effects model showed that asthma has no significant associations (OR = 1.58; p = 0.225) with NERD compared to the non-NERD group. Publication bias was noted from the funnel plot [Figure 5b]. Sensitivity analysis was performed and suggested that two studies13,15 should be excluded, and its publication bias was changed to low (Egger’s test, p = 0.134), and the heterogeneity test was found not significant (Q = 4.55; p = 0.103) [Figure 5c]. Therefore, a fixed-effects model (I2 = 56.05%) was used for three studies, and showed that having asthma has 2.53 times (OR = 2.53, 95% CI: 1.94–3.20; p < 0.001) significantly higher than those without asthma on NERD than the non-NERD group.
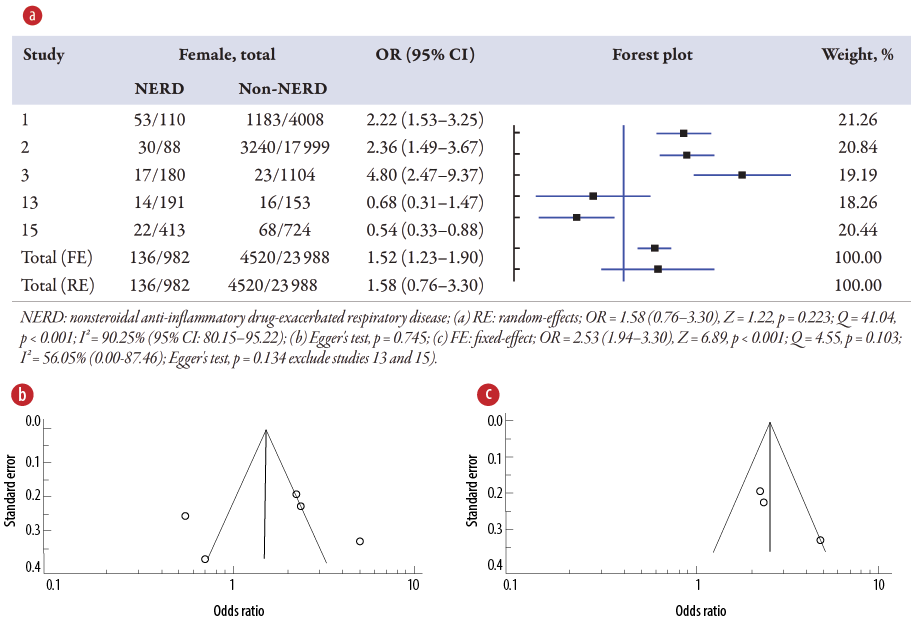 Figure 5: (a) Overview of the meta-analysis results of asthma on NERD and non-NERD groups.
Figure 5: (a) Overview of the meta-analysis results of asthma on NERD and non-NERD groups.
(b) Funnel plot for five studies. (c) Funnel plot for three studies after excluding two studies with publication bias.
Discussion
NSAIDs are commonly used for their anti-inflammatory effects. However, in some people with underlying respiratory conditions, taking NSAIDs can lead to a specific syndrome called NERD. NERD involves a combination of three symptoms: asthma, chronic sinus inflammation with nasal polyps, and respiratory reactions triggered by consuming NSAIDs. A meta-analysis has found that females, those with allergies, smokers, and those with a family history of asthma are more likely to develop NERD.
Female and risk of NERD
Gender appears to influence the risk of developing NERD, with females being more susceptible to the condition compared to males. Hormonal differences between males and females may play a role in modulating the immune response, thereby influencing the risk of NERD. Hormonal fluctuations, specifically the effects of progesterone and estrogen, are thought to be a factor in the higher risk of developing NERD in females. These hormones have been associated with inflammation and bronchial hyperresponsiveness (BHR), which could potentially affect the severity of respiratory symptoms triggered by NSAIDs.
Although it is mostly linked to female reproductive function, estrogen can have effects outside of the reproductive system. Even though the precise mechanisms are still being investigated, estrogen has been found to affect several physiological processes, including those connected to the respiratory system, such as influencing BHR through several mechanisms. Firstly, estrogen alters the immune system and causes inflammation in several tissues, including the airways. Inflammation is a major factor in BHR in asthma. Pro-inflammatory chemicals like cytokines and chemokines are produced more readily when estrogen is present, and this can lead to the onset or worsening of BHR.11,12 Secondly, estrogen has an impact on the smooth muscle’s capability to contract. Elevated estrogen concentrations have been linked to heightened smooth muscle contraction in the airways, potentially resulting in airway constriction and escalation of BHR. Calcium channels and contractile proteins are two examples of substances involved in smooth muscle contraction whose synthesis or activity may be influenced by estrogen.13,14
In addition to the hormone estrogen, the hormone progesterone can also impact BHR. Specifically, progesterone has been found to worsen airway inflammation and hyperresponsiveness to methacholine in animal models of allergic asthma. This is due to progesterone’s ability to exacerbate the airway’s interleukin-5-mediated eosinophilia, which is a key feature of allergic asthma.15 Progesterone may work by modifying the signaling pathways, such as calcium channels or contractile proteins, that are involved in the contraction of smooth muscle. Progesterone also interacts with estrogen and influences the BHR.16
In addition to estrogen and progesterone, elevated testosterone is also associated with a decreased likelihood of current asthma. However, the specific influence of testosterone on BHR is not well understood and may be complex. One theory for how testosterone might affect BHR is that it has anti-inflammatory effects. Given that inflammation is a significant factor in the development of BHR, testosterone has been demonstrated to have anti-inflammatory effects in various tissues. Therefore, by lowering airway inflammation, testosterone may have a protective effect against BHR.17
Having atopy and risk of NERD
Atopy, characterized by enhanced airway sensitivity, allergic inflammation, and mast cell activation, likely influences the risk of developing NERD through several mechanisms, as follows. Firstly, people who are atopic frequently have hyperresponsiveness and increased airway sensitivity, which makes them more vulnerable to respiratory stimuli. When exposed to NSAIDs, this heightened sensitivity may raise the chance of developing NERD.18 Secondly, an excessive response by the immune system to allergens in people with atopy may leads to persistent inflammation of the airways.18 This pre-existing inflammation may create a pro-inflammatory environment in the airways, making individuals more vulnerable to NSAID-induced respiratory reactions seen in NERD. Thirdly, increased mast cell activation, which is essential to allergies and NERD, is linked to atopy.19,20 Histamine and leukotrienes, two mediators that cause bronchoconstriction and inflammation, are released by mast cells. Atopy may intensify the mast cell reaction to NSAID exposure, exacerbating respiratory symptoms.19
Ever smoking and risk of NERD
Smoking is a known risk factor for the onset and progression of respiratory diseases like asthma and NERD, and it has a substantial effect on BHR and NERD. The following are some ways that smoking affects BHR. Firstly, smoking leads to chronic inflammation of the airways, which then contributes to increased BHR in NERD. It is already known that the toxic substances in tobacco smoke, including nicotine and other chemicals, can trigger an immune response in the respiratory system. Then, the inflammation can cause airway narrowing, increased mucus production, and structural changes in the airways, all of which contribute to BHR.21 Secondly, the production of excess mucus in the airways stimulated by smoking can clog the airways, hinder mucus clearance, and worsen the BHR.21 Thirdly, the process of airway remodeling, which is brought on by prolonged smoking, can cause structural alterations in the airways which then can worsen NERD. These alterations include smooth muscle hypertrophy, increased collagen deposition, and thickening of the airway walls, which will narrow the airways and lessen their capacity to dilate in response to different stimuli.23 Fourthly, smoking gradually deteriorates the lungs, which lowers lung function. Decreased lung function, including poorer gas exchange and reduced airflow, can aggravate and initiate BHR.23,24
Family history of asthma and risk of NERD
BHR can be influenced by an asthmatic family history.25 Exaggerated airway constriction or narrowing in response to various stimuli, including allergens, exercise, cold air, or irritants, is known as BHR. It is an attribute specific to asthma. In particular, the level of responsiveness to histamine in infants was related to the presence or absence of a family history of asthma. This finding suggests that the initial level of airway responsiveness may be genetically determined.26
Numerous genes are linked to a higher risk of developing asthma. These genes influence numerous biological processes, such as immune regulation, inflammation of the airways, and smooth muscle function in the airways. These genetic variants may be more likely to be inherited by people with a family history of asthma and may exacerbate BHR.27–29
Conclusion
The meta-analysis aimed to identify risk factors associated with the NERD. NERD is characterized by hypersensitivity to aspirin or NSAIDs, resulting in a chronic eosinophilic, inflammatory respiratory disorder in patients with asthma and chronic rhinosinusitis with nasal polyps. The study systematically reviewed the literature, identifying 19 eligible studies. The quality of the studies was assessed using the Joanna Briggs Institute Qualitative Assessment and Review Instrument. Descriptive statistics and meta-analysis were performed to estimate the pooled OR and identify risk factors associated with NERD. The analysis considered factors such as age, gender, body mass index, family history, smoking, and atopy. The risk factors for NERD found were being female, having atopy (enhanced airway sensitivity and allergic inflammation), a history of smoking, and a family history of asthma. Females are more susceptible to NERD due to hormonal differences and the effects of progesterone and estrogen on inflammation and BHR. Atopy increases the risk of NERD through heightened airway sensitivity, persistent inflammation, and increased mast cell activation. Smoking contributes to NERD by causing chronic airway inflammation, excess mucus production, airway remodeling, and decreased lung function. A family history of asthma indicates a genetic predisposition to BHR.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
references
- 1. Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy 2019 Jan;74(1):28-39.
- 2. Fawzy A, Putcha N, Aaron CP, Bowler RP, Comellas AP, Cooper CB, et al; SPIROMICS Investigators. SPIROMICS Investigators. Aspirin use and respiratory morbidity in COPD: a propensity score-matched analysis in subpopulations and intermediate outcome measures in COPD study. Chest 2019 Mar;155(3):519-527.
- 3. Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID-exacerbated respiratory disease: a meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy 2015 Jul;70(7):828-835.
- 4. Al-Saleh JA, Saab MA, Negm A, Balushi F, Namas R, Ziade N. Predictors of not achieving remission or low disease activity in axial spondyloarthritis patients from middle eastern countries: a prospective, multicenter, real-world study. Oman Med J 2022 May;37(3):e375.
- 5. Hannawi SM, Hannawi H, Al Salmi I. Cardiovascular risk in rheumatoid arthritis: literature review. Oman Med J 2021 May;36(3):e262.
- 6. White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med 2018 Sep;379(11):1060-1070.
- 7. Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2002 Nov;89(5):474-478.
- 8. Makowska JS, Burney P, Jarvis D, Keil T, Tomassen P, Bislimovska J, et al. Respiratory hypersensitivity reactions to NSAIDs in Europe: the global allergy and asthma network (GA2 LEN) survey. Allergy 2016 Nov;71(11):1603-1611.
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009 Aug;151(4):W65-94.
- 10. JBI. Critical appraisal tools. [2025 January 11]. Available from: https://jbi.global/critical-appraisal-tools.
- 10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003 Sep;327(7414):557-560.
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986 Sep;7(3):177-188.
- 12. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007 Aug;28(5):521-574.
- 13. Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol 2017 Sep;199(5):1573-1583.
- 14. Bhallamudi S, Connell J, Pabelick CM, Prakash YS, Sathish V. Estrogen receptors differentially regulate intracellular calcium handling in human nonasthmatic and asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2020 Jan;318(1):L112-L124.
- 15. Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 2012 Nov;303(10):L923-L928.
- 16. Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 2003 Oct;33(10):1457-1463.
- 17. Frump AL, Lahm T. Sex hormone signaling in the lung in health and disease: airways, parenchyma, and pulmonary vasculature. In: Hemnes AR, editor. Gender, sex hormones and respiratory disease: a comprehensive guide. Cham: Springer International Publishing; 2016. p. 27-62.
- 18. Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 2011 Apr;76(4):585-588.
- 19. Suh DI, Koh YY. Relationship between atopy and bronchial hyperresponsiveness. Allergy Asthma Immunol Res 2013 Jul;5(4):181-188.
- 20. Kuruvilla ME, Vanijcharoenkarn K, Levy JM. The role of mast cells in aspirin-exacerbated respiratory disease (AERD) pathogenesis: implications for future therapeutics. J Asthma Allergy 2020 Oct;13:463-470.
- 21. Theoharides TC, Tsilioni I, Ren H. Recent advances in our understanding of mast cell activation - or should it be mast cell mediator disorders? Expert Rev Clin Immunol 2019 Jun;15(6):639-656.
- 22. Chalmers GW, MacLeod KJ, Thomson L, Little SA, McSharry C, Thomson NC. Smoking and airway inflammation in patients with mild asthma. Chest 2001 Dec;120(6):1917-1922.
- 23. Chethana R, Mishra P, Kaushik M, Jadhav R, Dehadaray A. Effect of smoking on nasal mucociliary clearance. Indian J Otolaryngol Head Neck Surg 2022 Oct;74(Suppl 2):956-959.
- 24. Hough KP, Curtiss ML, Blain TJ, Liu RM, Trevor J, Deshane JS, et al. Airway remodeling in asthma. Front Med (Lausanne) 2020 May;7:191.
- 25. Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy 2015 Apr;45(4):706-719.
- 26. Donath H, Klenner H, Hutter M, Meoli A, Trischler J, Schulze J, et al. Severe bronchial hyperresponsiveness along with house dust mite allergy indicates persistence of asthma in young children. Pediatr Allergy Immunol 2023 Dec;34(12):e14047.
- 27. Young S, Le Souëf PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med 1991 Apr;324(17):1168-1173.
- 28. Thomsen SF. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J 2015 Jan;2(Jan):2.