Postpartum hemorrhage (PPH) remains a leading cause of maternal mortality worldwide.1 PPH accounts for 8% of maternal deaths in developed countries and 32% in developing countries.1 The incidence of PPH ranges 3–8% of all deliveries.2–4
According to the World Health Organization (WHO), PPH is defined as blood loss exceeding 500 mL after vaginal delivery and 1000 mL after cesarean section.5,6 In 2017, the American College of Obstetricians and Gynecologists defined PPH as blood loss > 1000 mL or blood loss accompanied by hypovolemic signs and symptoms within 24 hours of delivery, regardless of delivery mode.6 The Royal College of Obstetricians and Gynaecologists defined minor PPH as blood loss between 500 and 1000 mL and major PPH as blood loss > 1000 mL.7 As the volume of estimated blood loss is unreliable, the general state of the patient can also be considered in defining PPH.8
The WHO recommends a visual estimate of blood loss as a standard for measurement. With the publication of the E-MOTIVE trial in 2023,9 the WHO confirmed that visual estimates corelates with other estimates like gravimetry and blood collection devices, although other studies have stated visual estimates underestimate blood loss by 33–50% compared to spectrometry.10,11 Other methods, such as gravimetry, blood collection devices, and hemodynamic assessments, have been proposed as alternatives.12–18
Identifiable risk factors for PPH include advanced maternal age, increased parity, history of previous PPH, uterine overdistention, placenta previa or accreta, and placental abruption. Intrapartum risk factors include induced labor, prolonged labor, and instrumental delivery, but most cases occur unexpectedly. Etiology for PPH has been suggested as four Ts: tone, trauma, tissue, and thrombin.19 Uterine atony accounts for 70% of PPH cases, often associated with prolonged labor, chorioamnionitis, uterine overdistention, macrosomia, and polyhydramnios.20
Mortality and severe maternal morbidity after PPH can result from shock, acute respiratory distress syndrome, disseminated intravascular coagulation, acute kidney injury, and pituitary necrosis. Anticipating, preventing, and early recognition with timely interventions has been consistently shown to reduce PPH morbidity and mortality.21,22
Although PPH continues to be the main cause of maternal death, it can be prevented by antenatally optimizing hemoglobin (Hb), correcting anemia, anticipating PPH in high-risk pregnancies, keeping cross-matched blood ready, and multidisciplinary team preparation in cases of placenta accreta spectrum including cell salvage equipment. In addition, intrapartum recognition and treatment chorioamnionitis, judicious use of oxytocin over prolonged periods, post-delivery practicing active management of the third stage universally, which includes use of oxytocic at delivery, controlled cord traction, and uterine massage. Ongoing training for healthcare providers and regular PPH drills are crucial for improving maternal outcomes. This study aimed to assess PPH prevalence, risk factors, and outcomes after vaginal and cesarean deliveries at Sultan Qaboos University Hospital (SQUH).
Methods
This cross-sectional study was conducted from January 2017 to December 2021 at SQUH, a tertiary healthcare institution in Oman. SQUH is a teaching hospital in Muscat, with 600 beds, 12 delivery suites, and 25 obstetric beds; 60% of patients delivering are booked, and many of them referred as high-risk patients needing tertiary care. About 40% are unbooked for labor and deliver. Ethical approval was obtained from the College of Medicine and Health Sciences Medical and Research Ethics Committee (MERC # 2850). PPH was defined as blood loss exceeding 500 mL after vaginal delivery and 1000 mL after cesarean section, per WHO criteria.5,6 In our delivery ward, we have visual charts that depict the blood loss when standard-size mops and drapes are soaked with blood, which is used in estimating blood loss after vaginal delivery. In addition, clots are weighed. During cesarean section, blood-soaked mops are weighed and blood in the suction bottles are measured.
All women delivering in SQUH had active management of the third stage of labor. During the study period, Syntometrine was used as the oxytocic agent after vaginal delivery and oxytocin after cesarean sections. Controlled cord traction and uterine massage were used after all deliveries.
When PPH is diagnosed, the management protocol includes calling for help, obtaining intravenous (IV) access, IV fluid administration, and uterotonic administration in atonic PPH along with looking for and treating traumatic PPH. A modified early obstetric warning score chart is maintained to monitor and decide on interventions. Regular obstetric drills and emergency obstetric workshops are conducted to reeducate and retrain midwives and doctors in managing emergencies like PPH.
Data were collected from the hospital’s patient information system (TrakCare) and the delivery ward registry. Maternal demographics (age, parity, and history of cesarean section), pre-delivery Hb levels, delivery mode, estimated blood loss, neonatal birth weight, and PPH management (medications used, blood transfusion, surgical procedures) were recorded. Hb at discharge was also recorded. Statistical analysis was performed using SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp).
Frequency tables and pie charts were used to display the prevalence of different study parameters. The chi-square test was used to test the association between the different risk factors and the need for blood transfusion. The percentages, frequencies, and categorical variables were reported. A p-value < 0.05 was considered statistically significant.
Results
A total of 18 136 vaginal deliveries were reported during the study period; 729 women had PPH giving a prevalence of 4.0% [Figure 1]. The causes of PPH included uterine atony in 616 (84.5%), trauma in 61 (8.4%), and retained placenta tissues in 52 (7.1%) women. There were no reported cases of blood clotting (thrombus) factors [Figure 2].
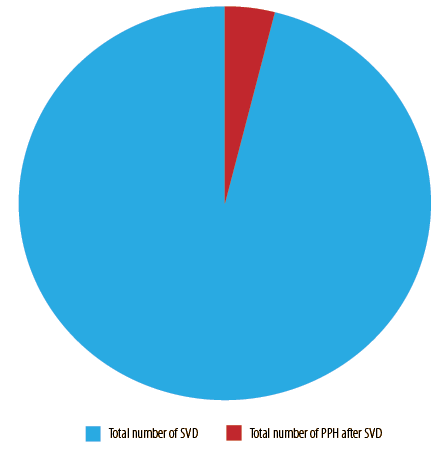 Figure 1: Prevalence of postpartum hemorrhage (PPH) after spontaneous vaginal delivery (SVD).
Figure 1: Prevalence of postpartum hemorrhage (PPH) after spontaneous vaginal delivery (SVD).
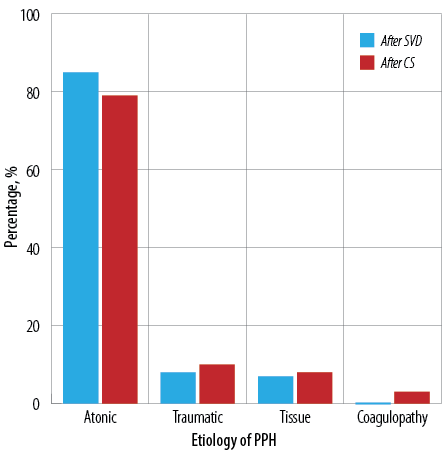 Figure 2: Etiology of postpartum hemorrhage (PPH) after spontaneous vaginal delivery (SVD) and cesarean section (CS).
Figure 2: Etiology of postpartum hemorrhage (PPH) after spontaneous vaginal delivery (SVD) and cesarean section (CS).
The mean maternal age among our women with PPH after vaginal delivery was 31.0 ± 12.3 (17–45) years. PPH cases were most prevalent in the age group 20–30 years, with 383 (52.5%) cases, followed by 31–40 years, with 312 (42.8%) cases. In the > 40 years group, there were 28 (3.8%) cases and six (0.8%) cases in < 20-year-olds.
Among the PPH cases, 174 (23.9%) women were primigravida, 244 (33.5%) were para 2–4, and 311 (42.7%) were para 5 and more. Out of the 729 PPH cases, 608 (83.4%) had a spontaneous vaginal delivery, including those who were born before arrival to the hospital (n = 28 (3.8%) cases), while 119 (16.3%) had instrumental delivery, the majority being vacuum deliveries. Two (0.3%) women had breech delivery.
The mean estimated blood loss was 860.0 ± 498.5 mL (500–5000 mL) [Table 1]. The neonatal birth weights were categorized into three main groups: > 4 kg, 2.5–4 kg, and < 2.5 kg. The number of neonates weighing > 4 kg was 13 (1.8%), 646 (88.6%) neonates weighed between 2.5–4 kg, and 70 (9.6%) were < 2.5 kg.
Table 1: Patients’ demographics after SVD.
|
Mean maternal age, years
|
31.0
|
|
Parity
|
|
|
Primiparous
|
174
|
|
2–4
|
244
|
|
≥ 5
|
311
|
|
Mean birth weight, kg
|
3.2
|
|
Previous CS
|
|
|
1
|
223
|
|
2–3
|
67
|
|
≥ 4
|
0
|
|
Hb level before delivery, g/dL
|
|
|
< 9
|
33
|
|
9–11
|
245
|
|
> 11
|
451
|
|
Hb level after delivery, g/dL
|
|
|
< 9
|
221
|
|
9–11
|
333
|
|
> 11
|
175
|
SVD: spontaneous vaginal delivery; CS: cesarean section;
Hb: hemoglobin.
The Hb level (pre- and post-delivery) was collected for all women and categorized into three groups: > 11, 9–11, and < 9 g/dL. The Hb before delivery was > 11 g/dL in 451 (61.9%), 9–11 g/dL in 245 (33.6%), and > 9 g/dL in 33 (4.5%) women. About 38.0% of the patients had a predelivery Hb of > 11 g/dL. On the other hand, the Hb level at discharge was found to be > 11 g/dL in 175 (24.0%), 9–11 g/dL in 333 (45.7%), and < 9 g/dL in 221 (30.3%) women.
Figure 3a shows the management of PPH, including 649 (89.0%) women who received pharmacological treatment. This was divided into four different groups. The first group included 430 (59.0%) women who received Syntometrine and oxytocin. The second group was those who received three uterotonics: syntometrine, oxytocin, and misoprostol, and this was noted in 193 (26.5%) women. The last group included 34 (4.7%) women who received four uterotonics: syntometrine, oxytocin, misoprostol, and carboprost. Overall, there were 164 (22.5%) women who received a combination of surgical and pharmacological management.
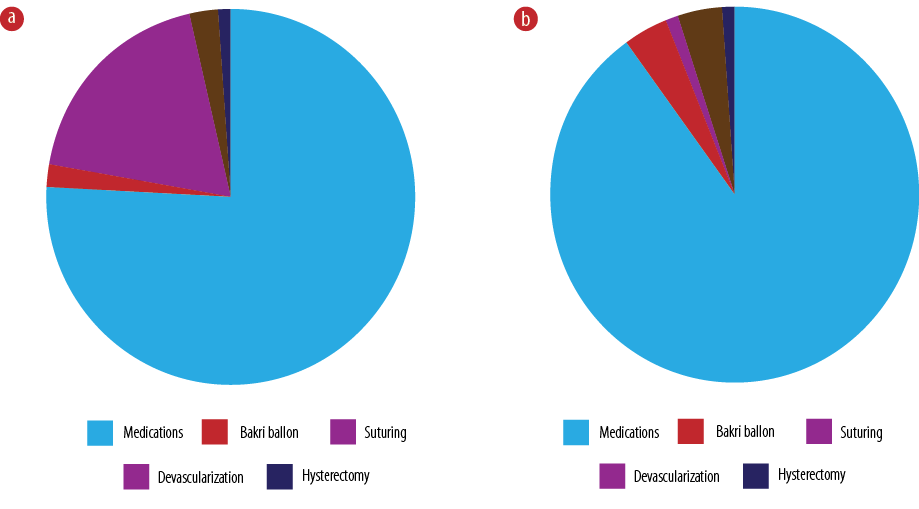 Figure 3: Management of postpartum hemorrhage (a) after spontaneous vaginal delivery and (b) cesarean section.
Figure 3: Management of postpartum hemorrhage (a) after spontaneous vaginal delivery and (b) cesarean section.
Surgical intervention was required in 244 (33.5%) women. Suturing for vaginal, cervical, and perineal tears was required in 162 (22.2%), manual removal of placenta in 21 (2.9%), and a Bakri balloon was inserted in 17 (2.3%) women. Twenty-seven (3.7%) women underwent examination under anesthesia. Nine (1.2%) women had vaginal packs, and only eight (1.1%) had hysterectomy. A vaginal pack was required to apply pressure as the vaginal tissue was very friable and edematous. Twenty-two (3.0%) women underwent devascularization procedures, either internal iliac artery or uterine artery ligation. Sixty-two (8.5%) women received packed red blood cell (PRBC), and 12 (1.6%) received PRBCs and other blood products such as fresh frozen plasma and cryoprecipitate.
The maternal mortality rate due to PPH after vaginal delivery was 0.1% (one patient). The patient had atonic PPH which was managed with four uterotonics, Bakri balloon, devascularization, and hysterectomy.
Of 2771 cesarean sections, 360 (13.0%) women had PPH [Figure 4]. The most common cause was uterine atony in 263 (79.0%), followed by traumatic PPH in 33 (9.9%), placental causes in 27 (8.1%), and coagulopathy in 10 (3.0%) women [Figure 2A].
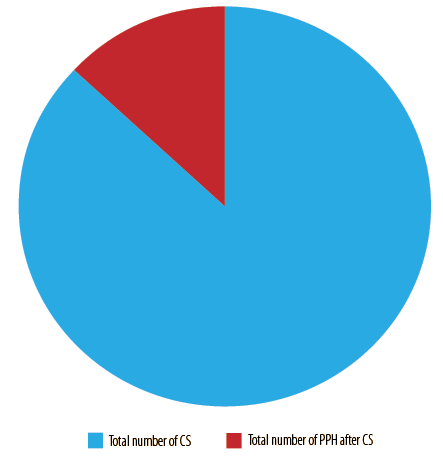 Figure 4: Prevalence of postpartum hemorrhage (PPH) after cesarean section (CS).
Figure 4: Prevalence of postpartum hemorrhage (PPH) after cesarean section (CS).
The mean maternal age of women with PPH after cesarean section was 34.0 ± 5.06 (19–47 years). In this group of women, 106 (29.4%) were primigravida, 175 (48.6%) were para 2–4, and 79 (21.9%) were para 5 and more. Previous cesarean sections accounted for 38.9% (n = 140) of cases [Table 2]. Macrosomia was the least common risk factor in this study sample, accounting for 3.1% (n = 11) of cases.
Table 2: Patients’ demographics after CS.
|
Mean maternal age, years
|
34.0
|
|
Parity
|
|
|
Primiparous
|
106
|
|
2–4
|
175
|
|
≥ 5
|
79
|
|
Mean birth weight, kg
|
2.9
|
|
Previous CS
|
|
|
1
|
55
|
|
2–3
|
56
|
|
≥ 4
|
18
|
|
Hb level before delivery, g/dL
|
|
|
< 9
|
12
|
|
9–11
|
128
|
|
> 11
|
220
|
|
Hb level after delivery, g/dL
|
|
|
< 9
|
188
|
|
9–11
|
156
|
|
> 11
|
16
|
CS: cesarean section; Hb: hemoglobin.
The number of neonates weighing > 4 kg was 11 (3.1%), 254 (70.6%) neonates were weighed between 2.5–4 kg, and 95 (26.4%) were < 2.5 kg. The mean estimated blood loss was 1400.0 mL±0.6 (1000–5500 mL) [Table 2]. The Hb before delivery was > 11 g/dL in 220 (61.1%) women, 9–11 g/dL in 128 (35.6%) women, and < 9 g/dL in 12 (3.3%) women. The Hb level at discharge was > 11 g/dL in 16 (4.4%) women, 9–11 g/dL in 156 (43.3%) women, and < 9 g/dL in 188 (52.2%) women.
Tranexamic acid and uterotonics were used to manage PPH in 300 (90.1%) women, and the Bakri balloon in 13 (4.0%) women. Thirteen (4.0%) women needed devascularization procedures, three (1.0%) women required compression sutures, and a further three (1.0%) needed hysterectomy. No interventional radiology, including uterine artery embolization, was required.
Oxytocin alone was used in 133 (40.0%) of cases, and two and three uterotonics were required in 35 (10.5%) and 30 (8.3%) cases, respectively. However, only 12 (3.6%) cases required a combination of four uterotonics. The findings of this study showed that most of the women did not require blood transfusion (n = 233, 64.7%), while the other 35.3% (n = 127) required blood transfusion. Among those who required blood transfusion, 78.7% (n = 100) received only PRBC, and 15.7% (n = 20) received a combination of PRBC, fresh frozen plasma, and platelets. While 5.5% required cell saver (n = 7).
Discussion
The prevalence of PPH after vaginal delivery was 4.0%. This was in concurrence with world literature of 3–8%.2–4 There are no studies reported on the prevalence of PPH in Oman. PPH after cesarean section was higher (13.0%), compared to normal vaginal deliveries. Cesarean births, especially emergency cesareans, remain a risk factor for both PPH and severe PPH.23,24 It is well known that method of birth is shown to be an important risk factor for minor PPH and may be a better predictor of PPH than antenatal risk factors.25 This higher prevalence with cesarean section could be because of a larger number of multiple previous cesarean sections, which is a high risk for PPH due to placental abnormalities and adhesions. The other reason could be a more objective measurement of blood loss by measuring blood in the suction device and weighing mops. The risk factors for severe PPH after cesarean deliveries include previous cesarean.21,26 Our study found the rates of previous scars to be 38.9%, in patients who had PPH after cesarean.
The other risk factors positively correlated with PPH after cesarean section include antepartum hemorhage,21,24 twins,23 preeclampsia,21 general anesthesia,23,24 anemia,21 and maternal age > 35.27
The prevalence of anemia in our study was 38.1%, which was higher than most studies that reported between 25–30%.28 There is a need for introspection on this account, as to why women, after receiving antenatal care, present to the delivery ward with anemia. This could be attributed to high parity, repeated pregnancies, and lactation. In addition, antenatal care should include checking compliance with oral iron preparations and reviewing if the prenatal vitamins routinely given to pregnant women who are not anemic at the start of their pregnancy have sufficient iron content to prevent anemia.
Taking steps for anemia correction before delivery will benefit our patients and reduce the PPH rate. Anemia can be a cause and a consequence of PPH. Reduced myoglobin in the myometrial fibers predisposes to atonicity. A smaller amount of blood loss can hemodynamically compromise an anemic patient.
The etiology of PPH after vaginal deliveries was 84.5% atonicity, which was similar to larger studies.2,4,29 Trauma accounted for 8.4% of our PPH cases, and 7.1% was due to retained tissue. There were no cases where PPH was attributed to coagulation abnormalities. Trauma and coagulation abnormalities were found as an etiology for PPH in around 20% and 1% in other studies.19
The etiology for PPH after cesarean section was comparable to other studies, which quote the incidence to be 84%,10%, 5.5%, and 0.3% for atony, trauma, placental abnormalities, and coagulation abnormalities, respectively.2,29 The coagulation abnormalities in our study were higher than in other literature. Advanced maternal age, also thought to be a risk factor,2,28,29 was not found to be common in our women with PPH. This could be explained by the fact that most of the women delivering at our institution were at a younger age.
The prevalence of PPH increased from 0.3% in nulliparous women to 2% in women with parity greater than four,2 which correlated with our PPH rates after vaginal delivery.
Active management of the third stage of labor has been shown to reduce the prevalence of PPH substantially.30,31 Active management of the third stage includes oxytocic at delivery of the anterior shoulder and controlled cord traction (when trained birth attendants are available). Early cord clamping is not recommended. This was strictly adhered to in our population. The E-MOTIVE intervention included M (uterine massage), O (oxytocic), T (tranexamic acid), IV (IV fluids), and E (examination and escalation).9 The oxytocic that is used in our institution is Syntometrine after vaginal delivery, if not contraindicated, and 20 units of oxytocin infusion after cesarean section. We have introduced carbetocin for all cesarean sections as a preventive measure and better utilization of tranexamic acid as there is good evidence of the effectiveness of both in reducing PPH.32–35
Macrosomia causes the uterus to be overdistended and increases the risk of atonic PPH. The incidence of macrosomia is increasing due to obesity and gestational diabetes mellitus.36,37
In patients with PPH after vaginal delivery and cesarean section, the percentage of babies with a birth weight of > 4 kg were 1.8 and 3.1%, respectively. Hence, macrosomia as an etiology for PPH was not high. Macrosomia has been identified as a risk factor for PPH in studies from Uganda and Tanzania.38,39 Uterine overdistention, causing thinning and poor myometrial contraction can cause PPH.39
Blood products were transfused in 84 and 120 patients after vaginal delivery and cesarean section, respectively. We utilized cell saver in 5.5% of our cesarean section patients with PPH. These were in patients with the placenta accreta spectrum. Having the availability of blood products and such resources can improve maternal outcomes. Improving healthcare services by allocating sufficient resources including human resources would contribute to significantly reducing the rate of PPH.40
Our study was limited by the small sample size and its retrospective nature. We did not look at whether the patients who had PPH after vaginal delivery were patients who had vaginal birth after cesarean section. Additionally, we did not address induced labor, prolonged labor, multiple pregnancies, or preeclampsia as risk factors.
Conclusion
The prevalence of PPH was 4.0% after vaginal delivery and 13.0% after cesarean delivery, comparable to international rates. The prevalence was high after cesarean section due to various risk factors. We noted a high rate of anemic women when admitted for delivery. Addressing anemia during pregnancy and further research on tranexamic acid and carbetocin use for cesarean sections are recommended to improve maternal outcomes.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014 Jun;2(6):e323-e333.
- 2. Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol 2010 Apr;202(4):353.e1-353.e6.
- 3. Mehrabadi A, Liu S, Bartholomew S, Hutcheon JA, Kramer MS, Liston RM, et al; Maternal Health Study Group of the Canadian Perinatal Surveillance System (Public Health Agency of Canada). Temporal trends in postpartum hemorrhage and severe postpartum hemorrhage in Canada from 2003 to 2010. J Obstet Gynaecol Can 2014 Jan;36(1):21-33.
- 4. Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013 Nov;209(5):449.e1-449.e7.
- 5. Kerr RS, Weeks AD. Postpartum haemorrhage: a single definition is no longer enough. BJOG 2017 Apr;124(5):723-726.
- 6. Committee on Practice Bulletins-Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol 2017 Oct;130(4):e168-e186.
- 7. Prevention and management of postpartum haemorrhage: green-top guideline no. 52. BJOG 2017 Apr;124(5):e106-e149.
- 8. Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol 2015 Jul;213(1):76.e1-76.e10.
- 9. Gallos I, Devall A, Martin J, Middleton L, Beeson L, Galadanci H, et al. Randomized trial of early detection and treatment of postpartum hemorrhage. N Engl J Med 2023 Jul;389(1):11-21.
- 10. Al Kadri HM, Al Anazi BK, Tamim HM. Visual estimation versus gravimetric measurement of postpartum blood loss: a prospective cohort study. Arch Gynecol Obstet 2011 Jun;283(6):1207-1213.
- 11. Schorn MN. Measurement of blood loss: review of the literature. J Midwifery Womens Health 2010;55(1):20-27.
- 12. Mavrides E, Allard S, Chandraharan E, Collins P, Green L, Hunt BJ, et al; Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. BJOG 2017 Apr;124(5):e106-e149.
- 13. Sentilhes L, Vayssière C, Deneux-Tharaux C, Aya AG, Bayoumeu F, Bonnet MP, et al. Postpartum hemorrhage: guidelines for clinical practice from the French college of gynaecologists and obstetricians (CNGOF): in collaboration with the French society of anesthesiology and intensive care (SFAR). Eur J Obstet Gynecol Reprod Biol 2016;198:12-21.
- 14. RANZCOG. Category: clinical guidance statement. Management of postpartum hemorrhage (PPH). 2021 [cited 2021 August 11]. Available from: https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Management-of-Postpartum-Haemorrhage-(C-Obs-43)-Review-July-2017.pdf?ext=.pdf.
- 15. Sri Lanka College of Obstetrician and Gynecologists. SLCOG guideline on management of primary postpartum haemorrhage. [cited 2021 August 11]. Available from: https://www.slcog.lk/wp-content/uploads/2021/02/SLCOG-Guideline-on-Management-of-Primary-Post-Partum-Haemorrhage-03.-2020.pdf.
- 16. Shields LE, Goffman D, Caughey A; Committee on Practice Bulletins-Obstetrics. Practice bulletin no.183: postpartum hemorrhage. Obstet Gynecol 2017 Oct;130(4):e168-e186.
- 17. Schlembach D, Helmer H, Henrich W, von Heymann C, Kainer F, Korte W, et al. Peripartum haemorrhage, diagnosis and therapy. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 015/063, March 2016). Geburtshilfe Frauenheilkd 2018 Apr;78(4):382-399.
- 18. Fawcus S. Alerts for managing postpartum haemorrhage. S Afr Med J 2018;108(12):1013-1017.
- 19. Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician 2007 Mar;75(6):875-882.
- 20. Oyelese Y, Ananth CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol 2010 Mar;53(1):147-156.
- 21. Kawakita T, Mokhtari N, Huang JC, Landy HJ. Evaluation of risk-assessment tools for severe postpartum hemorrhage in women undergoing cesarean delivery. Obstet Gynecol 2019 Dec;134(6):1308-1316.
- 22. Obstetric Care Consensus No; American College of Obstetricians and Gynecologists. 5: Severe maternal morbidity: screening and review. Obstet Gynecol 2016 Sep;128(3):e54-e60.
- 23. Rossen J, Okland I, Nilsen OB, Eggebø TM. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand 2010 Oct;89(10):1248-1255.
- 24. Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J 2005 Jul;98(7):681-685.
- 25. Hawker L, Weeks A. Postpartum haemorrhage (PPH) rates in randomized trials of PPH prophylactic interventions and the effect of underlying participant PPH risk: a meta-analysis. BMC Pregnancy Childbirth 2020 Feb;20(1):107.
- 26. Du L, Feng L, Bi S, Zhang L, Tang J, Zhong L, et al. Probability of severe postpartum hemorrhage in repeat cesarean deliveries: a multicenter retrospective study in China. Sci Rep 2021 Apr;11(1):8434.
- 27. Mitta K, Tsakiridis I, Dagklis T, Grigoriadou R, Mamopoulos A, Athanasiadis A, et al. Incidence and risk factors for postpartum hemorrahge: a case control study in a tertiary hospital in Greece. Medicina (Kaunas) 2023 Jun;59(6):1151.
- 28. Omotayo MO, Abioye AI, Kuyebi M, Eke AC. Prenatal anemia and postpartum hemorrhage risk: a systematic review and meta-analysis. J Obstet Gynaecol Res 2021 Aug;47(8):2565-2576.
- 29. Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG 2008 Sep;115(10):1265-1272.
- 30. Muir HA. Pharmacologic intervention for managing uterine atony and related maternal hemorrhage: what is the most effective drug dose? Can J Anaesth 2013 Nov;60(11):1047-1053.
- 31. Escobar MF, Nassar AH, Theron G, Barnea ER, Nicholson W, Ramasauskaite D et al. FIGO recommendation on management of postpartum hemorrhage. Int J Gynaecol Obstet 2022 Mar;157 Suppl 1(Suppl 1):3-50.
- 32. Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2015 Mar;3(3):CD007412.
- 33. Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012 Apr;2012(4):CD005457.
- 34. World Health Organization. WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. 2017 [cited 2021 August 11]. Available from: https://www.who.int/publications/i/item/9789241550154.
- 35. Roberts I, Brenner A, Shakur-Still H. Tranexamic acid for bleeding: much more than a treatment for postpartum hemorrhage. Am J Obstet Gynecol MFM 2023 Feb;5(2S):100722.
- 36. Zheng J, Xiao XH, Zhang Q, Mao LL, Yu M, Xu JP, et al. Correlation of placental microbiota with fetal macrosomia and clinical characteristics in mothers and newborns. Oncotarget 2017 Jul;8(47):82314-82325.
- 37. Rossi AC, Mullin P, Prefumo F. Prevention, management, and outcomes of macrosomia: a systematic review of literature and meta-analysis. Obstet Gynecol Surv 2013 Oct;68(10):702-709.
- 38. Ononge S, Mirembe F, Wandabwa J, Campbell OM. Incidence and risk factors for postpartum hemorrhage in Uganda. Reprod Health 2016 Apr;13:38.
- 39. Said AS, Manji KP. Risk factors and outcomes of fetal macrosomia in a tertiary centre in Tanzania: a case-control study. BMC Pregnancy Childbirth 2016 Aug;16(1):243.
- 40. Prapawichar P, Ratinthorn A, Utriyaprasit K, Viwatwongkasem C. Maternal and health service predictors of postpartum hemorrhage across 14 district, general and regional hospitals in Thailand. BMC Pregnancy Childbirth 2020 Mar;20(1):172.