Chronic hepatitis B virus (HBV) infection, being associated with substantial morbidity and mortality, has become a global health challenge.1–3 Approximately 257.5 million individuals, or 3.2% of the world population, live with chronic HBV infection, leading to around 555 000 deaths annually due to related complications.2,3
The course of chronic HBV infection varies among patients, resulting in a broad range of disease severity.4 A significant proportion of these patients have hepatitis B e antigen (HBeAg)-negative chronic HBV infection. These asymptomatic individuals are classified as inactive HBV carriers.5
According to the 2017 clinical practice guidelines of the European Association for the Study of the Liver (EASL), an inactive HBV carrier state is identified by the absence of HBeAg and the presence of the HBe antibody, along with consistently normal alanine aminotransferase (ALT) levels, and low or undetectable serum levels of HBV DNA (< 2000 IU/mL).6 However, serum HBV DNA levels may fluctuate up to 20 000 IU/mL in some cases.6 Inactive carriers generally have a better prognosis than active HBV-infected patients.5,7 However, their evolving clinical profile necessitates regular monitoring for HBV reactivation. Rarely, life-threatening complications such as hepatocellular carcinoma (HCC) and cirrhosis can occur, underscoring the importance of frequent screening of these patients.8,9
In practice, antiviral therapy tends to be delayed for inactive HBV carriers with normal ALT levels and minimal hepatic damage, resulting in uncertainty about when to start treatment.10,11 Untreated patients have a higher risk of cirrhosis and HCC, highlighting the need for early identification of fibrosis and close surveillance of at-risk individuals.12–16 While liver biopsy remains the gold standard for evaluating hepatic fibrosis, its invasive nature renders it suboptimal for routine monitoring of inactive HBV carriers.14 Therefore, international guidelines recommend non-invasive techniques such as serum markers and elastography for the initial staging and monitoring of fibrosis.16
In Oman, where HBV endemicity has been estimated at approximately 2–7%, research on inactive HBV carriers and their associated complications is lacking.1,17,18 Therefore, this study aimed to assess the occurrence and risk factors of significant hepatic fibrosis among Omani adults who are inactive HBV carriers.
Methods
This single-center retrospective study focused on adult Omani patients diagnosed as inactive HBV carriers using two-dimensional shear-wave elastography (2D SWE) at Sultan Qaboos University Hospital from January 2017 to December 2018. Ethical approval was obtained from the Medical Research and Ethics Committee of the College of Medicine and Health Sciences, Sultan Qaboos University (MREC #1548).
Potential participants’ data was gathered from the hospital’s electronic patient information system. We used the EASL (2017) definition for an inactive HBV carrier.6 The collected information included demographic data including age, sex, marital status, place of residence, and body mass index. Potential risk factors for fibrosis development were noted. These included family history of HBV, HCC, or cirrhosis, in addition to comorbidities such as type 2 diabetes mellitus, sickle cell disease, thalassemia, dyslipidemia, renal disease, heart failure, fatty liver disease, and malignancy. Nosocomial history, such as hemodialysis, organ transplantation, surgery, and blood transfusions, were also included. Lastly, various biochemical parameters were assessed, including platelet and white blood cell counts, and levels of ALT, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, albumin, total bilirubin, and alpha-fetoprotein.
Exclusion criteria were patients whose files had significant missing data, pregnant women, patients with concurrent chronic liver diseases such as hepatitis C, hepatitis D, HIV infections, autoimmune hepatitis, cholesteric liver disease, alpha-1 antitrypsin deficiency, Wilson’s disease, and hemochromatosis.
All patients had undergone simultaneous 2D SWE and liver ultrasound imaging under non-fasting conditions using the GE Logiq E9 2.0 with XDClear multi-purpose ultrasound system (GE Healthcare, Milwaukee, Wisconsin, USA). Changes in echotexture were assessed to diagnose cirrhosis and fatty liver disease and to classify the degree of hepatic fibrosis. Liver stiffness measurements (LSMs) were used to assign various stages of fibrosis, as follows: (1) F0: no fibrosis (≤ 7.1 kPa), (2) F1: minimal fibrosis (7.1–7.8 kPa), (3) F2: significant fibrosis (7.8–8.0), (4) F3: severe fibrosis (8.0–11.5 kPa), and (5) F4: cirrhosis (> 11.5 kPa). Stage F2 and higher were taken as indicators of significant hepatic fibrosis.19
The participants were divided into two groups: with significant hepatic fibrosis and without significant hepatic fibrosis [Figure 1]. The groups were compared to determine the potential risk factors for developing significant hepatic fibrosis in inactive HBV carriers.
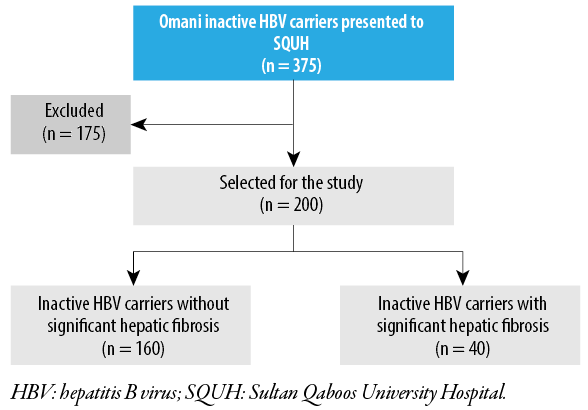 Figure 1: Flow diagram of the study population.
Figure 1: Flow diagram of the study population.
Data analysis was performed using SPSS Statistics (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). A chi-squared test was performed to assess associations between significant hepatic fibrosis development and potential risk factors. The normality of data distribution for continuous variables was evaluated using the Kolmogorov-Smirnov test. Parametric variables were analyzed using the student’s t-test, while non-parametric variables were assessed using the Mann-Whitney U test. Multivariate adjusted analysis utilized logistic regression, with variables exhibiting p-values < 0.25 in the crude analysis included in the regression model. A p-value < 0.05 was considered statistically significant.
Results
The subjects comprised 200 inactive HBV carriers with Omani nationality, among whom 106 (53.0%) were male. The participants’ mean age was 44.6 ± 9.3 (range = 31–79) years. Most (61.0%) were in the 40–60 years age group [Table 1]. None of our participants developed decompensated liver cirrhosis or HCC.
The distribution of patients across liver fibrosis stages, as determined by LSMs, was as follows: stage F0 = 142, F1 = 18, F2 = 3, F3 = 31, and F4 = 6 patients. Overall, 40 (20.0%) patients developed significant hepatic fibrosis, defined as stage F2 fibrosis or higher [Figure 2].
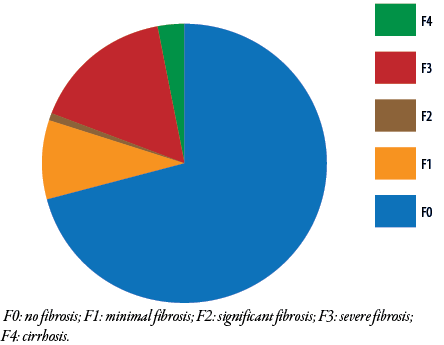 Figure 2: Fibrosis staging among Omani patients with hepatitis B e antigen-negative chronic hepatitis B virus infection (N = 200).
Figure 2: Fibrosis staging among Omani patients with hepatitis B e antigen-negative chronic hepatitis B virus infection (N = 200).
Approximately 58.5% of the cohort were overweight (body mass index > 25 kg/m2). Furthermore, 21 (10.5%) subjects had type 2 diabetes mellitus and 115 (57.5%) had non-alcoholic fatty liver disease (NAFLD) [Table 1]. Several risk factors were significantly associated with the presence of significant hepatic fibrosis in the studied population [Table 1 and Figure 3].
Table 1: Baseline characteristics of Omani patients with HBeAg-negative chronic HBV infection and associations with significant hepatic fibrosisa (N = 200).
|
Age group, years
|
|
|
|
|
|
|
30–40
|
63 (31.5)
|
9 (14.3)
|
54 (85.7)
|
5.618 (2.347–13.450)
|
0.003*
|
|
40–60
|
122 (61.0)
|
23 (18.9)
|
99 (81.1)
|
|
|
|
> 60
|
15 (7.5)
|
8 (53.3)
|
7 (46.7)
|
|
|
|
Sex
|
|
|
|
|
|
|
Male
|
106 (53.0)
|
33 (31.1)
|
73 (68.9)
|
1.114 (0.451–2.755)
|
< 0.001*
|
|
Female
|
94 (47.0)
|
7 (7.4)
|
87 (92.6)
|
|
|
|
BMI > 25 kg/m2
|
117 (58.5)
|
22 (18.8)
|
95 (81.2)
|
0.786 (0.152–4.074)
|
1.000
|
|
T2DM
|
21 (10.5)
|
7 (33.3)
|
14 (66.7)
|
1.074 (0.336–3.432)
|
0.145
|
|
Dyslipidemia
|
19 (9.5)
|
4 (21.1)
|
15 (78.9)
|
-
|
1.000
|
|
HTN
|
17 (8.5)
|
9 (52.9)
|
8 (47.1)
|
5.516 (1.974–15.416)
|
0.002*
|
|
NAFLD
|
115 (57.5)
|
29 (25.2)
|
86 (74.8)
|
2.268 (1.060–4.853)
|
0.049*
|
|
Renal disease
|
5 (2.5)
|
3 (60.0)
|
2 (40.0)
|
6.405 (1.033–39.718)
|
0.056
|
|
Family history
|
|
|
|
|
|
|
HBV infection
|
58 (29.0)
|
9 (15.5)
|
49 (84.5)
|
1.150 (0.230–5.762)
|
0.413
|
|
HBV-related cirrhosis
|
22 (11.0)
|
2 (9.1)
|
20 (90.9)
|
2.212 (0.828–5.911)
|
0.259
|
|
HBV-related HCC
|
9 (4.5)
|
2 (22.2)
|
7 (77.8)
|
0.366 (0.082–1.635)
|
1.000
|
|
Hemoglobin, g/dL†
|
13.0 ± 1.8
|
13.7 ± 1.6
|
12.9 ± 1.8
|
-
|
0.009*
|
|
Platelet count × 109/L†
|
243.5 ± 67.8
|
218.4 ± 70.1
|
249.7 ± 65.9
|
-
|
0.009*
|
|
White blood cell count × 109/L†
|
5.5 ± 1.9
|
5.2 ± 2.0
|
5.5 ± 1.9
|
-
|
0.150
|
|
ALT, U/L†
|
22.5 ± 11.5
|
26.4 ± 13.7
|
21.5 ± 10.7
|
-
|
0.027
|
|
AST, U/L†
|
19.7 ± 6.2
|
20.9 ± 7.0
|
19.4 ± 5.9
|
-
|
0.079
|
|
ALP, U/L†
|
66.5 ± 19.2
|
73.2 ± 26.4
|
64.8 ± 16.5
|
-
|
0.061
|
|
Albumin, g/L†
|
44.9 ± 3.4
|
45.2± 3.4
|
44.8 ± 3.4
|
-
|
0.520
|
|
Total bilirubin, µmol/L†
|
9.0 ± 6.5
|
9.9 ± 5.6
|
8.8 ± 6.7
|
-
|
0.063
|
|
AFP, ng/mL†
|
2.5 ± 1.9
|
2.2 ± 1.7
|
2.5 ± 2.1
|
-
|
0.979
|
HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; BMI: body mass index; T2DM: type 2 diabetes mellitus; HTN: hypertension; NAFLD: non-alcoholic fatty liver disease; HCC: hepatocellular carcinoma; ALT: alanine transaminase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; AFP: alpha-fetoprotein. aDefined as liver stiffness measurements of > 7.8 kPa. *Significance;†Expressed as mean ± SD.
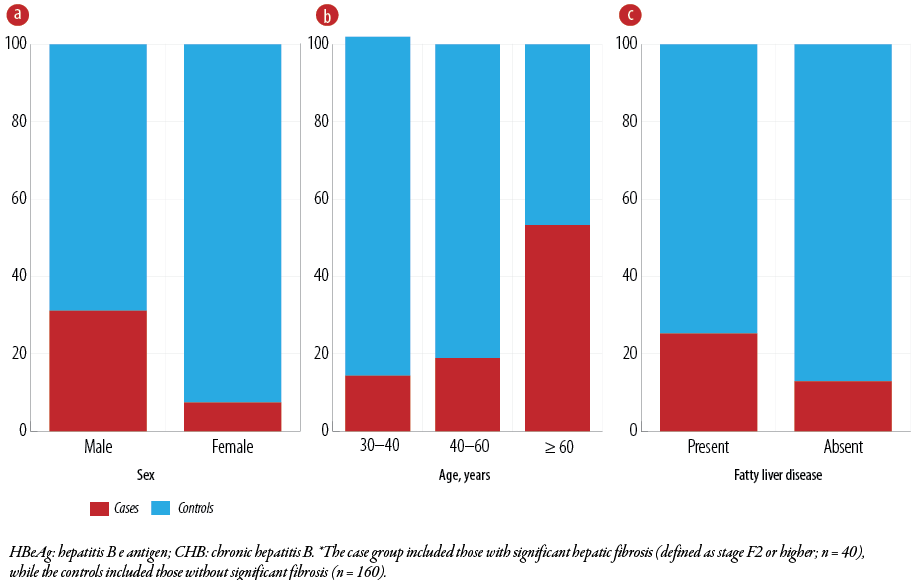 Figure 3: Associations between significant hepatic fibrosis* and (a) sex (p < 0.001), (b) age (p = 0.003), and (c) fatty liver disease (p = 0.049) among Omani patients with HBeAg-negative CHB infection (N = 200).
Figure 3: Associations between significant hepatic fibrosis* and (a) sex (p < 0.001), (b) age (p = 0.003), and (c) fatty liver disease (p = 0.049) among Omani patients with HBeAg-negative CHB infection (N = 200).
Men were significantly more likely to develop significant fibrotic changes (31.1%) than women (7.4%) (OR = 1.114, 95% CI: 0.451–2.755; p < 0.001) [Figure 3a]. Patients aged ≥ 60 years were also more likely to have significant fibrosis (53.3%) than those aged 40–60 years (18.9%) and 30–40 years (14.3%) [Figure 3b]. Univariate analysis revealed that old patients (≥ 60 years) had a five-fold higher risk of significant fibrosis compared to younger patients (p = 0.003) [Table 1].
Regarding comorbidities, hypertension (OR = 5.516, 95% CI: 1.974–15.416; p = 0.002) and NAFLD (OR = 2.268, 95% CI: 1.060–4.853; p = 0.049) were significantly correlated with significant liver fibrosis [Figure 3c]. Table 1 shows that 52.9% of hypertensive patients and 25.2% of those with NAFLD had significant fibrosis.
The biochemical parameters positively associated with significant hepatic fibrosis were higher hemoglobin levels (p = 0.009) and higher ALT levels (p = 0.027). Platelet count (p = 0.009) was negatively associated. [Table 1] Multivariate regression analysis of all participants unveiled independent associations of sex, age, and NAFLD with significant hepatic fibrosis, as determined by LSMs [Table 2].
Table 2: Multivariate analysis of risk factors for developing significant fibrosis among Omani patients with HBeAg-negative chronic HBV infection (N = 200).
|
Age group, years
|
-
|
0.072
|
|
40–60
|
1.329 (0.478–3.695)
|
0.585
|
|
> 60
|
10.900 (1.371–86.631)
|
0.024*
|
|
Sex
|
6.568 (1.673–25.785)
|
0.007*
|
|
HTN
|
1.659 (0.248–11.093)
|
0.601
|
|
NAFLD
|
2.650 (1.025–6.855)
|
0.044*
|
|
T2DM
|
0.360 (0.066–1.977)
|
0.240
|
|
Chronic kidney disease
|
6.538 (0.499–85.592)
|
0.152
|
|
Hemoglobin level
|
0.939 (0.678–1.300)
|
0.703
|
|
Platelet count
|
0.993 (0.986–1.000)
|
0.060
|
|
ALT level
|
1.016 (0.979–1.055)
|
0.404
|
|
ALP level
|
1.016 (0.996–1.037)
|
0.117
|
*Significance; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; HTN: hypertension; NAFLD: nonalcoholic fatty liver disease; T2DM: type 2 diabetes mellitus; ALT: alanine transaminase; ALP: alkaline phosphatase.
Men were sixfold more likely to develop significant fibrosis (p = 0.007) than women. Patients aged > 60 years had a tenfold increased risk than those aged 30–40 years (p = 0.024). NAFLD emerged as an independent risk factor for fibrosis (p = 0.044) [Table 2].
Discussion
Our study aimed to evaluate the prevalence and associated factors for developing significant hepatic fibrosis using non-invasive methods in Omani patients diagnosed as inactive HBV carriers. One-fifth (20.0%) of the participants manifested significant hepatic fibrosis. Male sex, age ≥ 60 years, and the presence of NAFLD were found to be independently associated with significant fibrosis.
Studies from countries with low HBV endemicity, such as France and Italy have indicated that inactive HBV carriers generally demonstrated good prognoses, with a minimum prevalence of HBV-related hepatic fibrosis.20,21 A Chinese study also reported similarly.22 High and intermediate endemic regions have a higher prevalence of such complications.23–25 For example, a longitudinal, cross-sectional study from Taiwan, which has an intermediate-to-high HBV prevalence, found that 16.1% of inactive HBV carriers experienced viral reactivation, while 15% developed hepatic fibrosis over a 25-year period.15 A study from Egypt, an intermediate HBV prevalence country, reported that 20% of inactive HBV carriers exhibited significant fibrotic changes as determined by liver biopsy.24
The Omani population is considered to have an intermediate HBV prevalence. To the best of our knowledge, the present study is the first in Oman to shed light on the prevalence of inactive HBV carriers and patients with significant fibrosis, using non-invasive measures. Thus, our results provide essential baseline data, which could help in the early detection of fibrosis and facilitate optimal management of this subpopulation. This is also one of the pioneering studies focusing on inactive HBV carriers in the Arabian Gulf region.
The overall prevalence of significant fibrosis (20.0%) among Omani inactive HBV carriers identified in the current study aligned with findings reported from other intermediate HBV endemic areas (15–20%).15,24 In contrast, Saudi Arabia has reported a higher prevalence rate of 30.9%.26 A study in Hong Kong has found a 33.3% prevalence.27 However, the higher prevalence rates in the last two studies may partly be attributed using liver biopsies instead of 2D SWE for hepatic fibrosis staging. Meanwhile, a study in Spain, an intermediate HBV-endemic country, adopted a non-invasive elastography technique like ours and found a more comparable prevalence of 25%.28 The observed discrepancies in prevalence rates might also be attributable to factors such as different LSM criteria (> 7.5 vs. > 7.8 kPa), ultrasound equipment, and study durations.
The present study also investigated the risk factors of fibrosis. Significant associations were observed between stage F2 or higher levels of fibrosis and sex and age. Our findings are consistent with reports from Taiwan and Bangladesh, where both male sex and advanced age were significantly linked to the development of hepatic fibrosis in inactive HBV carriers.15,29 On the other hand, studies from Spain and Hong Kong did not find significant associations with these two factors.27,28 An unexpected finding from a Taiwanese study was a significantly higher hepatic fibrosis risk among middle-aged patients (40–60 years) compared to older patients (≥ 60 years).30
In a recent study conducted in Oman, intra-familial transmission emerged as the predominant mode of HBV transmission, while nosocomial transmission was found to be relatively uncommon.18 Nonetheless, given the highly infective nature of HBV, the present study explored both nosocomial and family-related factors as possible risk factors for fibrotic changes. We found that a quarter of inactive HBV carriers with stage F2 fibrosis or higher had non-significant associations with a family history of chronic HBV infection, HCC, or cirrhosis. As other studies have also reported that individuals with a family history of HBV may have an increased risk of advanced liver scarring due to earlier-occurring and potentially longer-lasting infections,18,24 our findings, despite their low significance, seem to merit further research.
Regarding comorbidities, we found that only hypertension and fatty liver disease exhibited significant associations with significant fibrosis. Notably, there is a paucity of research exploring the association between hypertension and its impact on inactive HBV carriers. A 2014 literature review included only one study (from Spain), which assessed these factors, found non-significant associations with both hypertension and fatty liver disease.28
Though the coexistence of fatty liver disease and chronic HBV infection has been studied extensively, there is a dearth of studies illuminating the prevalence of such coexistence among inactive HBV carriers. A recent review highlighted a synergistic escalation of liver fibrosis risk when fatty liver disease coexists with chronic HBV infection, which is consistent with the observations of the present study.30 The mechanism behind this appears multifactorial, encompassing genetic, metabolic, and immune factors.30
The interest in non-invasive diagnostic tests for hepatic fibrosis in chronic liver diseases has grown considerably in recent decades. Such tests would mitigate the need for invasive procedures like liver biopsy and their associated complications, making regular follow-ups more viable. Researchers have explored the potential of various biochemical parameters as risk factors for fibrosis development and their efficacy in predicting such complications.31,32 As a notable example, previous research has revealed a significant correlation between elevated ALT levels and fibrosis development, suggesting that it may be a reliable predictor for fibrosis in inactive HBV carriers.32,33 The present study also found ALT levels to have a positive significant correlation with significant fibrosis. We must be cautious, however, as other studies have challenged the predictive accuracy of ALT regarding significant liver injury.24,27
We observed a negative correlation between platelet count and significant fibrosis. A similar negative correlation has also been reported in Chinese and Iranian populations, suggesting the potential utility of this variable for predicting hepatic fibrosis.27,34 A more recent study from China also identified a negative correlation between platelet count and significant fibrosis in chronic HBV patients.35 The biochemical findings described above support their potential utility as non-invasive tools for investigating fibrosis risk in Omani patients.
A unique finding of our study was a significant positive association between hemoglobin and fibrosis. However, similar findings were not found in the literature, which makes it an intriguing topic for further investigation.
This study has several strengths. Firstly, it marked the first documentation in Oman of the prevalence of liver fibrosis among inactive HBV carriers. Secondly, it assessed a diverse array of risk factors, thereby providing a more holistic understanding of this subset of patients. However, we encountered a few limitations. The primary constraint stemmed from the restricted timeframe, limiting the sample size. A larger sample would have enhanced the reliability of comparisons between the case and control groups. Additionally, the retrospective nature of the study resulted in missing data related to crucial biochemical parameters, such as high-density and low-density lipoproteins, thyroxine, and thyroid-stimulating hormone. Consequently, analysis of these variables as potential risk factors was not possible. Finally, the current research focused on a single center in Muscat, and any generalization of its findings requires caution. Future studies should seek to eliminate these shortcomings and recruit larger patient populations from different regions of Oman.
Conclusion
Our findings indicated that 20.0% of Omani inactive HBV carriers exhibited significant fibrosis, with male sex, age ≥ 60 years, and the presence of fatty liver on ultrasound being independent risk factors. These results underscored the critical need for periodic assessment and monitoring of inactive HBV carriers, particularly those with high-risk factors. Utilizing non-invasive tests for fibrosis can help detect disease progression early, allowing for timely intervention and better patient outcomes. Routine biochemical parameters such as platelet count, hemoglobin, and ALT levels could be helpful predictors of disease prognosis. The study provides valuable baseline data for understanding and managing significant fibrosis in this subpopulation, potentially aiding in early detection and optimal patient care. However, further research, including larger prospective studies, is recommended to validate these findings and comprehensively explore the inactive HBV carrier stage and its related complications.
Disclosure
The authors declare no conflicts of interest. No funding was received for this study.
references
- 1. Gao Y, Wang M, Liu X. Noninvasive serum markers for predicting significant liver histopathology in HBeAg-negative chronic HBV-infected patients with normal alanine aminotransferase. Microbiol Spectr 2024 Apr;12(4):e0394123.
- 2. GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol 2022 Sep;7(9):796-829.
- 3. Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol 2023 Oct;8(10):879-907.
- 4. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009 May;49(5)(Suppl):S45-S55.
- 5. Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J 2005 Sep;2:82.
- 6. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017 Aug;67(2):370-398.
- 7. Invernizzi F, Viganò M, Grossi G, Lampertico P. The prognosis and management of inactive HBV carriers. Liver Int 2016 Jan;36(Suppl 1):100-104.
- 8. Wei S, Xie Q, Liao G, Chen H, Hu M, Lin X, et al. Patients with chronic hepatitis B who have persistently normal alanine aminotransferase or aged < 30 years may exhibit significant histologic damage. BMC Gastroenterol 2024 Mar;24(1):120.
- 9. Tunçer G, Geyiktepe-Güçlü C, Bayramlar OF, Atasoy-Bozan B, Yücel Ç, Sürme S, et al. Predictors of significant histological hepatic abnormality in treatment-naive patients infected with HBeAg-negative chronic hepatitis B. Infect Dis Clin Microbiol 2024 Mar;6(1):22-31.
- 10. Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, et al. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat 2021 Jul;28(7):1025-1033.
- 11. Di Bisceglie AM, Lombardero M, Teckman J, Roberts L, Janssen HL, Belle SH, et al; Hepatitis B Research Network (HBRN). Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat 2017 Apr;24(4):320-329.
- 12. Choi GH, Kim GA, Choi J, Han S, Lim YS. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment Pharmacol Ther 2019 Jul;50(2):215-226.
- 13. Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut 2018 May;67(5):945-952.
- 14. Pita I, Horta-Vale AM, Cardoso H, Macedo G. Hepatitis B inactive carriers: an overlooked population? GE Port J Gastroenterol 2014 Nov-Dec;21(6):241-249.
- 15. Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009 Jul;104(7):1693-1699.
- 16. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. Electronic address: easloffice@easloffice.eu. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017 Jan;66(1):153-194.
- 17. Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004;38(10 Suppl 3):S158-S168.
- 18. Al-Busafi SA, Al-Harthi R, Al-Naamani K, Al-Zuhaibi H, Priest P. Risk factors for hepatitis B virus transmission in Oman. Oman Med J 2021 Jul;36(4):e287.
- 19. Sporea I, Bota S, Gradinaru-Taşcău O, Sirli R, Popescu A, Jurchiş A. Which are the cut-off values of 2D-Shear wave elastography (2D-SWE) liver stiffness measurements predicting different stages of liver fibrosis, considering transient elastography (TE) as the reference method? Eur J Radiol 2014 Mar;83(3):e118-e122.
- 20. Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Ollivier S, et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol 2002 Apr;36(4):543-546.
- 21. Delle Monache M, Petrelli A, Rossi A, Cecere R, Mirisola C, Costanzo G, et al. Noninvasive evaluation of liver fibrosis in a sample of putative inactive HBV carriers in Rome, Italy. Can J Infect Dis Med Microbiol 2021 Aug;2021:3068690.
- 22. Malik R, Kennedy P, Suri D, Brown A, Goldin R, Main J, et al. The role of liver fibrosis assessment in the management of patients with chronic hepatitis B infection: lessons learned from a single centre experience. Hepat Res Treat 2011;2011:524027.
- 23. Papatheodoridis GV, Manesis EK, Manolakopoulos S, Elefsiniotis IS, Goulis J, Giannousis J, et al. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology 2008 Nov;48(5):1451-1459.
- 24. Fateen AA, Shahin RY, Farres MN, Eldeeb MA, Amer HA. Assessment of hepatic fibrosis and necroinflammation among inactive HBsAg carriers in Egypt. Ann Hepatol 2012;11(4):464-470.
- 25. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008 May;134(5):1376-1384.
- 26. Sanai FM, Babatin MA, Bzeizi KI, Alsohaibani F, Al-Hamoudi W, Alsaad KO, et al. Accuracy of international guidelines for identifying significant fibrosis in hepatitis B e antigen–negative patients with chronic hepatitis. Clin Gastroenterol Hepatol 2013 Nov;11(11):1493-1499.e2.
- 27. Seto WK, Lai CL, Ip PP, Fung J, Wong DK, Yuen JC, et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B. PLoS One 2012;7(2):e32622.
- 28. Mena Á, Pedreira JD, Castro Á, López S, Vázquez P, Poveda E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol 2014 Jan;29(1):173-178.
- 29. Al Mahtab M, Akbar SM, Aguilar JC, Guillen G, Penton E, Tuero A, et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One 2018 Aug;13(8):e0201236.
- 30. Zhang J, Lin S, Jiang D, Li M, Chen Y, Li J, et al. Chronic hepatitis B and non-alcoholic fatty liver disease: conspirators or competitors? Liver Int 2020 Mar;40(3):496-508.
- 31. Zhou K, Gao CF, Zhao YP, Liu HL, Zheng RD, Xian JC, et al. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010 Sep;25(9):1569-1577.
- 32. Tan Y, Ye Y, Zhou X, Chen L, Wen D. Age as a predictor of significant fibrosis features in HBeAg-negative chronic hepatitis B virus infection with persistently normal alanine aminotransferase. PLoS One 2015 Apr;10(4):e0123452.
- 33. Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol 2012 Jul;57(1):196-202.
- 34. Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol 2006 Nov;101(11):2537-2545.
- 35. Ding R, Zheng J, Huang D, Wang Y, Li X, Zhou X, et al. INR-to-platelet ratio (INPR) as a novel noninvasive index for predicting liver fibrosis in chronic hepatitis B. Int J Med Sci 2021 Jan;18(5):1159-1166.