Malaria, a protozoan infection, is endemic to tropical countries and is responsible for a significant burden of disease and death, particularly in sub-Saharan Africa.1 The 2023 World Malaria Report recorded 249 million cases of malaria and 580 000 deaths.2 Though these numbers reflect an improvement from malaria indices recorded almost two decades ago, the 2023 World Malaria Report indicated that malaria continues to pose a significant public health challenge.2,3 Of the global malaria burden, Nigeria, along with 10 other countries, accounts for about 70% of cases and deaths. This situation prompted adopting the ‘high burden, high impact’ approach by the World Health Organization (WHO) and the Roll Back Malaria initiative in 2018.4 Despite these efforts, Nigeria reported an estimated 68 million cases of malaria and 194 000 deaths in 2021, accounting for 27% of the global disease burden.5
In Nigeria, as in many regions worldwide, a significant proportion of malaria-related deaths occur in children, particularly those under five years old, who comprise up to 80% of fatalities.5 These deaths are often due to severe forms of the disease, leading to end-organ damage and poor outcomes.6 The central nervous system is frequently affected during severe malaria, leading to diffuse encephalopathy with neurological manifestations, commonly referred to as ‘cerebral malaria’.7 Cerebral malaria is a significant complication of severe malaria in children, comprising 20%–75% of severe malaria cases.8–10 Additionally, 20%–30% of cerebral malaria survivors may have neurological complications at discharge, with some progressing to permanent neurological impairments.11,12
Although cerebral malaria is a common complication of severe malaria in children, the pathophysiological mechanisms underlying brain injuries and subsequent poor outcomes are not fully understood, necessitating further studies in both experimental animal models and clinical settings.7,12–14 Improving outcomes associated with cerebral malaria requires a thorough understanding of the contributing factors, particularly in resource-constrained settings. We aimed to describe the burden of childhood cerebral malaria and identify factors predictive of in-hospital mortality and short-term neurological complications.
Methods
This study retrospectively analyzed children diagnosed with cerebral malaria who were managed at a tertiary hospital in northwestern Nigeria from 1 January 2019 to 31 December 2022. The hospital has a 24-bed emergency pediatric complex that manages children with acute emergencies and receives referrals from within and outside Katsina state, northwestern Nigeria. We included children aged three months to 14 years with confirmed malaria based on a positive rapid diagnostic test or microscopic confirmation of Plasmodium falciparum and features of cerebral malaria. According to WHO criteria, cerebral malaria was defined based on the presence of the following: (1) impaired consciousness (Blantyre coma score < 3 or Glasgow coma score < 11) lasting ≤ 30 minutes; (2) malaria parasitemia (either a positive rapid diagnostic test or presence of P. falciparum under a blood film examination); and (3) the absence of any other plausible cause of the encephalopathy.15 We excluded patients with other forms of malaria without features of cerebral malaria,15 underlying chronic diseases, cancers, and pre-existing neurological abnormalities.
The minimum sample size for this study was estimated using the online sample size calculator Raosoft® (http://www.raosoft.com/samplesize.html). Using a prevalence of 5.6% of cerebral malaria obtained among children in a study in southwestern Nigeria, we obtained a minimum sample size of 224 at a 95% CI and a 3% margin of error.16
From electronic health records, we retrieved the following information about children with cerebral malaria (age, sex, duration of illness, presenting complaints, physical findings at admission, neurological sequels observed at discharge, dichotomous outcomes of discharge or death, follow-up date, and neurological status at follow-up), and neurological outcomes during follow-up were extracted and recorded as either fully recovered, recovering, or unknown neurological outcome (for those lost to follow-up).
The main outcomes were the variables at admission (clinical and laboratory features at hospitalization) that predicted hospitalization deaths and neurological complications. Secondary outcomes included the description of children profiles with cerebral malaria and recovery from neurological sequelae.
The data extracted were analyzed using SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Age (not normally distributed) was summarized using median and IQR and categorical variables were summarized using frequency tables. Chi-square and Fisher’s exact tests were used to examine the relationships between clinical features, laboratory findings (baseline at admission), and outcomes (hospitalization deaths, occurrence of neurological complications, and recovery at post-discharge). Variables significant in the bivariate analysis, along with age and sex, were entered into a binary logistic regression model, with non-survivors (hospitalization outcome) as the dependent variable. The binary logistic regression results were expressed as adjusted odds ratios (AORs) with 95% CI. Statistical significance was set at p < 0.05.
This study was conducted in accordance with the Declaration of Helsinki. The Federal Medical Centre Human Health Ethical Review Committee approved the study (FMCNHREC.REG.N003/0830441). As this was a retrospective study, the committee waived the requirement for informed consent. Data were extracted anonymously, with de-identification of the study children, and kept with absolute confidentiality.
Results
Of 8295 pediatric admissions during the four-year study period, 284 cases of cerebral malaria were identified with a prevalence of 3.4%. Of the 948 cases of severe pediatric malaria during the study period, 284 (30.0%) had cerebral malaria. The median age (IQR) of children with cerebral malaria was 3.0–8.0 years, higher than that of 4.0 (2.0–8.0) years for those children without cerebral malaria (U = 85 444.5, p = 0.017). Among the cerebral malaria cases, majority were male (164; 57.7%) and aged ≥ 5 years (160; 56.3%) [Table 1].
Table 1: Age and sex distribution of the study participants. Data is given as n (%).
|
< 1
|
3 (1.1)
|
2 (1.2)
|
1 (0.8)
|
|
1–< 5
|
121 (42.6)
|
72 (43.9)
|
49 (40.8)
|
|
5–10
|
126 (44.4)
|
68 (41.5)
|
58 (48.3)
|
Of the 284 cerebral malaria cases, 73.9% (n = 210) had only cerebral malaria, 10.2%(n = 29) had cerebral malaria and hemoglobinuria, and 2.1% (n = 6) had cerebral malaria and multiple other clinical features [Figure 1].
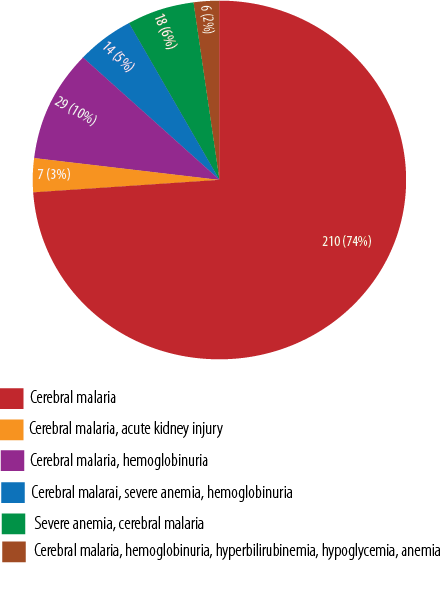 Figure 1: Spectrum of cerebral malaria along with other clinical features.
Figure 1: Spectrum of cerebral malaria along with other clinical features.
Most clinical features were comparable between survivors and non-survivors among children with cerebral malaria, except for the presence of hypoxemia (p = 0.016) and duration of loss of consciousness (p < 0.001). In addition, the length of hospitalization was shorter among the non-survivors (p < 0.001)
[Table 2]. Among the laboratory findings, variables that were significantly associated with hospitalization outcomes were the presence of acidosis, white blood cell counts, serum sodium, and serum creatinine [Table 3].
Table 2: Clinical and laboratory features (baseline) among children with cerebral malaria.
|
Age, years
|
< 5
|
124 (43.7)
|
107
|
17
|
0.631
|
0.464
|
|
≥ 5
|
160 (56.3)
|
143
|
17
|
|
|
|
Sex
|
Male
|
164 (57.7)
|
146
|
18
|
0.366
|
0.582
|
|
Female
|
120 (42.3)
|
104
|
16
|
|
|
|
Fever
|
Yes
|
266 (93.7)
|
234
|
32
|
0.014
|
1.000
|
|
No
|
18 (6.3)
|
16
|
2
|
|
|
|
Vomiting
|
Yes
|
51 (18.0)
|
46
|
5
|
0.277
|
0.812
|
|
No
|
233 (82.0)
|
204
|
29
|
|
|
|
Hypoxemia
|
< 90
|
19 (6.7)
|
13
|
6
|
7.428
|
0.016
|
|
≥ 90
|
265 (93.3)
|
237
|
28
|
|
|
|
Respiratory distress
|
Yes
|
69 (24.3)
|
58
|
11
|
1.363
|
0.286
|
|
No
|
215 (75.5)
|
192
|
23
|
|
|
|
Tachycardia
|
Yes
|
137 (48.2)
|
119
|
18
|
0.342
|
0.587
|
|
No
|
147 (51.8)
|
131
|
16
|
|
|
|
LOC
|
Yes
|
118 (41.5)
|
102
|
16
|
0.483
|
0.579
|
|
No
|
166 (58.5)
|
148
|
18
|
|
|
|
Duration of LOC, hours
|
≤ 24
|
68 (23.9)
|
47
|
21
|
30.339
|
< 0.001
|
|
> 24
|
216 (76.1)
|
203
|
13
|
|
|
|
Convulsions
|
Yes
|
193 (68.0)
|
169
|
24
|
0.123
|
0.846
|
|
No
|
91 (32.0)
|
81
|
10
|
|
|
|
LOH, hours
|
> 24
|
231 (81.3)
|
211
|
20
|
12.898
|
< 0.001
|
|
≤ 24
|
53 (18.7)
|
39
|
14
|
|
|
|
Acidosis*
|
< 15
|
53 (18.7
|
40
|
13
|
9.748
|
0.002
|
|
≥ 15
|
231 (81.3)
|
210
|
21
|
|
|
|
WBC, × 10/L
|
> 10
|
92 (32.4)
|
74
|
18
|
7.445
|
0.006
|
|
≤ 10
|
192 (67.6)
|
176
|
16
|
|
|
|
Neutrophils, %
|
≤ 60
|
162 (57.0)
|
140
|
22
|
0.926
|
0.362
|
|
> 60
|
122 (43.0)
|
110
|
12
|
|
|
|
Lymphocytes, %
|
≤ 40
|
156 (54.9)
|
138
|
18
|
0.062
|
0.804
|
|
> 40
|
128 (45.1)
|
112
|
16
|
|
|
|
PCV, %
|
≥ 15
|
263 (92.6)
|
231
|
32
|
0.129
|
1.000
|
|
< 15
|
21 (7.4)
|
19
|
2
|
|
|
|
Platelets, × 10/L
|
< 150
|
125 (44.0)
|
113
|
12
|
3.130
|
0.272
|
|
150–450
|
149 (52.5)
|
127
|
22
|
|
|
|
≥ 450
|
10 (3.5)
|
10
|
0
|
|
|
|
Sodium, mmol/L
|
≥ 125
|
194 (68.3)
|
178
|
16
|
8.058
|
0.005
|
|
< 125
|
90 (31.7)
|
72
|
18
|
|
|
|
Potassium, mmol/L
|
≤ 5.5
|
10 (3.5)
|
9
|
1
|
0.038
|
1.000
|
|
> 5.5
|
274 (96.5)
|
241
|
33
|
|
|
|
Cr, mg/dL
|
≤ 1.5
|
260 (91.5)
|
236
|
24
|
21.935
|
< 0.001
|
LOC: loss of consciousness; LOH: length of hospitalization; WBC: white blood cell; PCV: packed cell volume; Cr: creatinine.
*Bicarbonate < 15 mmol/L.
Table 3: Predictors (clinical and laboratory features) of hospitalization deaths among children with cerebral malaria.
|
Age, years
|
< 5
|
17
|
-0.494
|
0.421
|
1
|
|
0.241
|
|
≥ 5
|
17
|
|
|
0.610
|
0.267–1.393
|
|
|
Sex
|
Female
|
16
|
-0.136
|
0.439
|
1
|
|
0.789
|
|
Male
|
18
|
|
|
0.873
|
0.369–2.061
|
|
|
Hypoxemia
|
≥ 90
|
28
|
1.804
|
0.658
|
1
|
|
0.006
|
|
< 90
|
6
|
|
|
6.071
|
1.672–22.043
|
|
|
LOH, hours
|
> 24
|
20
|
1.781
|
0.457
|
1
|
|
< 0.001
|
|
≤ 24
|
14
|
|
|
5.934
|
2.423–14.535
|
|
|
Acidosis**
|
≥ 15
|
21
|
0.900
|
0.492
|
1
|
|
0.067
|
|
< 15
|
13
|
|
|
2.459
|
0.938–6.444
|
|
|
WBC, × 10/L
|
≤ 10
|
16
|
0.667
|
0.435
|
1
|
|
0.125
|
|
> 10
|
18
|
|
|
1.948
|
0.830–5.232
|
|
|
Sodium, mmol/L
|
≥ 125
|
16
|
0.806
|
0.433
|
1
|
|
0.062
|
|
< 125
|
18
|
|
|
2.240
|
0.959–5.232
|
|
|
Creatinine, mg/dL
|
≤ 1.5
|
24
|
1.905
|
0.579
|
1
|
|
< 0.001
|
AOR: adjusted odds ratio; LOH: length of hospitalization; WBC: white blood cell.
*Baseline at admission; **Bicarbonate < 15 mmol/L.
Of the 284 children, 34 deaths were recorded during hospitalization, with a case fatality rate of 12.0%. After adjusting for confounders, variables (baselines at admission) that predicted hospitalization deaths were the presence of hypoxemia (AOR = 6.071; 95% CI: 1.672–22.043) and baseline serum creatine > 1.5 mg/dL (AOR = 6.720; 95% CI: 2.160–20.905). Also, the first 24 hours of hospitalization were highly predictive of death (AOR = 5.934; 95% CI: 2.423–14.535) [Table 4].
Table 4: Neurological outcomes among children with cerebral malaria.
|
At discharge
|
|
|
|
|
|
|
Seizures
|
13 (26.5)
|
9 (36.0)
|
4 (16.7)
|
3.862
|
0.443
|
|
Motor deficit
|
11 (22.4)
|
4 (16.0)
|
7 (29.2)
|
|
|
|
Cortical blindness
|
10 (20.4)
|
4 (16.0)
|
6 (25.0)
|
|
|
|
Speech impairment
|
9 (18.4)
|
4 (16.0)
|
5 (20.8)
|
|
|
|
Cognitive impairment
|
6 (12.2)
|
4 (16.0)
|
2 (8.3)
|
|
|
|
At follow-up
|
|
|
|
|
|
|
Recovered
|
24 (49.0)
|
7 (28.0)
|
17 (70.8)
|
9.471
|
0.008
|
|
Still recovering
|
18 (36.7)
|
12 (48.0)
|
6 (25.0)
|
|
|
Of the 250 cerebral malaria survivors, 49 (19.6%) had neurological complications at discharge. The most common neurological complications were seizures (n = 13; 26.5%), followed by motor deficits (n = 11; 22.4%) and cognitive impairment (n = 6; 12.2%), as shown in Table 4. Among the clinical features, age and the presence of fever were associated with neurological complications at discharge from the hospital [Table 5]. For age, children < 5 years had higher odds of neurological complications (OR = 2.059; 95% CI: 1.094–3.876) compared with children ≥ 5 years. In contrast, children with fever had lower odds of neurological complications at discharge (OR = 0.281; 95% CI: 0.099–0.798). In contrast to clinical features, none of the laboratory features were significantly associated with neurological complications at discharge [Table 5].
Table 5: Factors associated with neurological complications at discharge.
|
Age, years
|
< 5
|
107 (42.8)
|
28
|
79
|
5.121
|
0.025
|
|
≥ 5
|
143 (57.2)
|
21
|
122
|
|
|
|
Sex
|
Male
|
146 (58.4)
|
25
|
121
|
1.366
|
0.261
|
|
Female
|
104 (41.6)
|
24
|
80
|
|
|
|
Fever
|
Yes
|
234 (93.6)
|
42
|
192
|
6.327
|
0.020
|
|
No
|
16 (6.4)
|
7
|
9
|
|
|
|
Vomiting
|
Yes
|
46 (18.4)
|
8
|
38
|
0.175
|
0.692
|
|
No
|
204 (81.6)
|
41
|
163
|
|
|
|
Hypoxemia
|
< 90
|
13 (5.2)
|
2
|
11
|
0.155
|
1.000
|
|
≥ 90
|
237 (94.8)
|
47
|
190
|
|
|
|
Respiratory distress
|
Yes
|
58 (23.2)
|
10
|
48
|
0.267
|
0.606
|
|
No
|
192 (76.8)
|
39
|
153
|
|
|
|
Tachycardia
|
Yes
|
131 (52.4)
|
25
|
106
|
0.047
|
0.874
|
|
No
|
119 (47.6)
|
24
|
95
|
|
|
|
LOC
|
Yes
|
102 (40.8)
|
20
|
82
|
0.000
|
1.000
|
|
No
|
148 (59.2)
|
29
|
119
|
|
|
|
Duration of LOC, hours
|
≤ 24
|
79 (31.6)
|
14
|
65
|
0.259
|
0.732
|
|
> 24
|
171 (68.4)
|
35
|
136
|
|
|
|
Duration of LOH, hours
|
≤ 24
|
39 (15.6)
|
9
|
30
|
0.354
|
0.518
|
|
> 24
|
211 (84.4)
|
40
|
171
|
|
|
|
Convulsions
|
Yes
|
169 (67.6)
|
35
|
134
|
0.408
|
0.611
|
|
No
|
81 (32.4)
|
14
|
67
|
|
|
|
Acidosis**
|
< 15
|
40 (16.0)
|
11
|
29
|
1.886
|
0.193
|
|
≥ 15
|
210 (84.0)
|
38
|
172
|
|
|
|
WBC, × 10/L
|
> 10
|
74 (29.6)
|
14
|
60
|
0.031
|
0.865
|
|
≤ 10
|
176 (70.4)
|
35
|
141
|
|
|
|
Neutrophils, %
|
≤ 60
|
140 (56.0)
|
28
|
112
|
0.032
|
0.874
|
|
> 60
|
110 (44.0)
|
21
|
89
|
|
|
|
Lymphocytes, %
|
≤ 40
|
138 (55.2)
|
25
|
113
|
0.431
|
0.526
|
|
> 40
|
112 (44.8)
|
24
|
88
|
|
|
|
PCV, %
|
≥ 15
|
231 (92.4)
|
45
|
186
|
0.028
|
0.772
|
|
< 15
|
19 (7.6)
|
4
|
15
|
|
|
|
Platelets, × 10/L
|
< 150
|
113 (45.2)
|
22
|
91
|
2.661
|
0.342
|
|
150–450
|
127 (50.8)
|
27
|
100
|
|
|
|
≥ 450
|
10 (4.0)
|
0
|
10
|
|
|
|
Sodium, mmol/L
|
≥ 125
|
178 (71.2)
|
37
|
141
|
0.552
|
0.488
|
|
< 125
|
72 (28.8)
|
12
|
60
|
|
|
|
Potassium, mmol/L
|
≤ 5.5
|
241 (96.4)
|
47
|
194
|
0.041
|
0.691
|
|
> 5.5
|
9 (3.6)
|
2
|
7
|
|
|
|
Cr, mg/dL
|
≤ 1.5
|
236 (94.4)
|
48
|
188
|
1.460
|
0.315
|
LOC: loss of consciousness; LOH: length of hospitalization; WBC: white blood cells ; PCV: packed cell volume; Cr: creatinine.
*Baseline at admission; **Bicarbonate <15 mmol/L.
At follow-up, 24 (49.0%) of the 49 children with neurological complications at discharge had fully recovered, whereas 18 (36.7%) were still recovering [Table 4]. The follow-up period after discharge ranged from 2–66 days, with a median of 6.5 (IQR = 4.3–9.0) days. In addition, follow-up after discharge revealed a significant relationship between recovery after neurological complications and sex [Table 4].
Discussion
Malaria continues to be a significant disease, disproportionately impacting Nigeria, with cerebral malaria being one of its most common severe forms. This study examined the prevalence of cerebral malaria among overall admissions and among those with severe malaria, with rates of 3.4% and 30.0%, respectively. Our findings were similar to the prevalence of cerebral malaria among all hospitalized children in Nigeria and Cameroon reported at 3.7% and 4.3%, respectively.10,17 A higher prevalence of 5.6% was observed among pediatric admissions in southwestern Nigeria,18 possibly because of differences in the study population and season of the study. In addition, our observed prevalence of cerebral malaria was lower than that reported in Mozambique (0.2% and 2.1% in pediatric admissions and severe malaria cases, respectively).19 The difference may be due to variations in the study population. All participants in our study had severe malaria, with slightly more than half being children aged ≥ 5 years. In contrast, nearly all children (91.4%) in the Mozambique study were under two years of age, and 73.0% had uncomplicated malaria. It is worth noting that despite the reported similar burden of cerebral malaria from total pediatric admission, a far higher proportion of cerebral out of all severe malaria cases (14.3%) was found in the study in Cameroon, with a larger percentage of under-fives (70.0%).17 Thus, the disparity from most of the aforementioned studies, when compared to ours, may be due to variations in age groups, immunogenicity of malaria parasites, genetic predisposition, and possibly malaria endemicity.20,21 Variances in host immune factors, such as cytokine profiles, genetic polymorphisms, and acquired immunity, dictate population responses to infestation and differences in childhood cerebral metabolic crisis.14 Populations with a more profound immunological response to severe malaria are at higher risk of cerebral malaria.
This study showed that hypoxemia, duration of loss of consciousness, duration of hospitalization, acidosis, white blood cell count, serum sodium, and serum creatinine were associated with hospitalization outcomes. These findings were comparable to those of a study conducted in southwestern Nigeria,18 where poor outcomes were associated with white blood cell count, hematocrit, platelet count, and acute kidney injury. Similarly, a study in Malawi among 1663 children with cerebral malaria documented relationships between age, loss of consciousness, and acidosis with poor outcomes.22 Also, in keeping with our observations, the authors documented a higher likelihood of neurological sequelae among younger age groups and those with longer durations of unconsciousness and the higher likelihood of death associated with acidosis.22
The case fatality rate of 12.0% in our study was far lower than the 18.0% and 28.8% reported by studies in Nigeria and Uganda, respectively.18,23 The lower case fatality rate found in our study may be related to the higher proportion of children older than five years and possibly the hospital policy of having at least the first dose of intravenous artesunate freely available for children diagnosed with severe malaria. Research has documented that the earlier artesunate is administered to children with severe malaria, the better the outcomes.21 This observation supports the need to make intravenous artesunate for severe malaria available free for children at pediatric hospitals or emergency points.
The predictors of hospitalization deaths were hypoxemia (AOR = 6.071), baseline serum creatinine > 1.5 mg/dL (AOR = 6.720), and the first 24 hours of hospitalization (AOR = 5.934). In Malawi,22 a significant relationship was observed between acidosis and mortality, while a review of predictors of outcomes of cerebral by malaria in another study identified impaired consciousness, hypoxemia, metabolic acidosis, and acute kidney injury as predictors of mortality in severe malaria.24 The six-fold likelihood of mortality within the first 24 hours in our patients was comparable to the substantial increase in mortality risk within the same timeframe observed in a multicenter study involving 7765 children with severe malaria.10 This finding suggested that the severity of the disease in patients may be higher in the first 24 hours of admission and a need for closer monitoring during the early period of hospitalization.
The prevalence of neurological complications at discharge was 19.6%, half of whom recovered during the follow-up. This was slightly less than reports of 23.1% and 22.0% prevalence of neurological complications among children with cerebral malaria in Malawi and Nigeria, respectively.18,25 Similar to our observations, the studies also observed varying neurodisabilities, including motor, sensory, and language deficits, and seizures among survivors of cerebral malaria, re-enforcing the impact of cerebral malaria on the children's brain.18,25 In contrast, the observed neurological complications in this study were far less than the 40.0% recorded in another Nigerian study,26 probably due to differences in the study sample and location. This study had 284 cases of childhood cerebral malaria in northern Nigeria compared with 20 cases studied in southwestern Nigeria.26 The study showed that under-fives were associated with increased odds of neurological complications at discharge, while fever at presentation confers lower odds of neurological complications. Studies have documented a higher likelihood of neurological complications in children under five compared to older children, probably a reflection of the increased susceptibility to neuronal damage.16,22,26
While this study has a relatively large sample size, there are some limitations. First, this single-center study does not reflect the whole country. Also, seven (14.3%) out of 49 children with neurological complications at discharge were lost to follow-up. In addition, we based our definition of cerebral malaria on the WHO definition criteria, which included a coma lasting 30 minutes or more, evidence of P. falciparum, and the absence of any other plausible cause of encephalopathy. Worthy of note is the fact that additional features through cranial imaging and retinal changes on fundoscopic examination have been found to improve the diagnosis of childhood cerebral malaria.7 However, in our facility, cranial imaging and fundoscopic examinations for retinal changes were not routinely carried out in children with cerebral malaria; hence, they were not included in the extracted data used for this study.
Conclusion
Cerebral malaria remains a significant cause of morbidity and mortality among children in northwestern Nigeria, with hypoxemia and elevated serum creatinine identified as critical predictors of in-hospital death. Children under five years old were particularly vulnerable to poor outcomes, including long-term neurological complications. Our findings highlighted the need for early diagnosis, prompt treatment, and close monitoring of children with cerebral malaria to improve outcomes.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014 Feb;383(9918):723-735.
- 2. World Health Organization. World malaria report 2023. 2023 [cited 2024 February 19]. Available from: https://www.who.int/publications/i/item/9789240086173.
- 3. Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria: global progress 2000 - 2015 and future challenges. Infect Dis Poverty 2016 Jun;5(1):61.
- 4. World Health Organization. High burden to high impact: a targeted malaria response. 2018 [cited 2024 February 19]. Available from: https://www.who.int/publications/i/item/WHO-CDS-GMP-2018.25.
- 5. World Health Organization. Report of malaria in Nigeria 2022. [cited 2024 February 19]. Available from: https://www.afro.who.int/countries/nigeria/publication/report-malaria-nigeria-2022-0.
- 6. Kafai NM, Odom John AR. Malaria in children. Infect Dis Clin North Am 2018 Mar;32(1):189-200.
- 7. Song X, Wei W, Cheng W, Zhu H, Wang W, Dong H, et al. Cerebral malaria induced by plasmodium falciparum: clinical features, pathogenesis, diagnosis, and treatment. Front Cell Infect Microbiol 2022 Jul;12:939532.
- 8. Orimadegun AE, Fawole O, Okereke JO, Akinbami FO, Sodeinde O. Increasing burden of childhood severe malaria in a Nigerian tertiary hospital: implication for control. J Trop Pediatr 2007 Jun;53(3):185-189.
- 9. Voloc A, Kamgaing EK, Ategbo S, Fleury J, Siawaya D. Outcomes of severe malaria and its clinical features in Gabonese children. Front Trop Dis 2022 Sep;3:985890 .
- 10. Adigun MB, Alao MA, Akindolire AE, Suleiman BM, Ibrahim OR. Determinants of outcomes of childhood severe malaria: a multi-centric study. Med J Armed Forces India 2023 Nov.
- 11. Postels DG, Chimalizeni YF, Mallewa M, Boivin MJ, Seydel KB. Pediatric cerebral malaria: a scourge of Africa Future Neurol 2013 Jan;8(1):67-85.
- 12. Guenther G, Muller D, Moyo D, Postels D. Pediatric cerebral malaria. Curr Trop Med Rep 2021 Jun;8(2):69-80.
- 13. Conroy AL, Datta D, John CC. What causes severe malaria and its complications in children? Lessons learned over the past 15 years. BMC Med 2019 Mar;17(1):52.
- 14. O’Brien NF, Chetcuti K, Fonseca Y, Vidal L, Raghavan P, Postels DG, et al. Cerebral metabolic crisis in pediatric cerebral malaria. J Pediatr Intensive Care 2023;2021 Aug;12(4):278-288.
- 15. World Health Organization. Guidelines for the treatment of malaria, 3rd ed. 2015 [cited 2024 February 19]. Available from: https://iris.who.int/handle/10665/162441.
- 16. Ayobami A, Ojuawo O, Onigbinde A, Joel-Medewase VI, Alabi GO. Clinical and haematological determinants of outcome among children with cerebral malaria in a tertiary centre in Nigeria. Niger J Paediatr 2022 Mar;49(1):1-7.
- 17. Chiabi A, Djimafo AN, Nguefack S, Mah E, Nguefack Dongmo F, Angwafo F III. Severe malaria in Cameroon: pattern of disease in children at the yaounde gynaeco-obstetric and pediatric hospital. J Infect Public Health 2020 Oct;13(10):1469-1472.
- 18. Alabi AO, Onigbinde MO, Ojuawo A, Joel-MedewASE VI, Alabi GO, Oladibu OT, et al. Bedside prognostic indicators of fatal outcome among children with cerebral malaria at a tertiary Nigerian hospital. Journal of Clinical and Diagnostic Research 2023 Jan 1;17(1):10-15.
- 19. Bassat Q, Guinovart C, Sigaúque B, Aide P, Sacarlal J, Nhampossa T, et al. Malaria in rural Mozambique. Part II: children admitted to hospital. Malar J 2008 Feb;7:37.
- 20. Tukwasibwe S, Mboowa G, Sserwadda I, Nankabirwa JI, Arinaitwe E, Ssewanyana I, et al. Impact of high human genetic diversity in Africa on immunogenicity and efficacy of RTS,S/AS01 vaccine. Immunogenetics 2023 Jun;75(3):207-214.
- 21. Sena-Dos-Santos C, Cavalcante GC, Marques D, Silva CS, de Moraes MR, Pinto P, et al. Association of apoptosis-related variants to malaria infection and parasite density in individuals from the Brazilian Amazon. Malar J 2023 Oct;22(1):295.
- 22. Borgstein A, Zhang B, Lam C, Gushu MB, Liomba AW, Malenga A, et al. Delayed presentation to hospital care is associated with sequelae but not mortality in children with cerebral malaria in Malawi. Malar J 2022 Feb;21(1):60.
- 23. Sande K. Evaluation of the risk factors and prevalence of cerebral malaria in children below 10 years in Kiryandogo general hospital. IDOSR J Sci Technol 2023 Mar;9(1):75-85.
- 24. Patel H, Dunican C, Cunnington AJ. Predictors of outcome in childhood Plasmodium falciparum malaria. Virulence 2020 Dec;11(1):199-221.
- 25. Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, et al. Blantyre malaria project epilepsy study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010 Dec;9(12):1173-1181.
- 26. Oninla S, Ogunro P, Oninla O, Kayode O. Childhood cerebral malaria in Nigeria: clinical features, treatment and outcome. Int J Trop Dis Health 2015 Feb;12(4):1-12.