Puberty is a complex coordinated developmental stage characterized by changes in a child’s body transforming it into an adult body with secondary sexual characteristics, reproductive capacity, gonadal maturation, and somatic growth.1–3 The exact onset of puberty depends on the central effect of the hypothalamic-pituitary-gonadal (HPG) axis and gonadotropin-releasing hormone (GnRH) pulse generator through multiple excitatory and inhibitory neuromodulators.4–6 Currently, there is a noticeable worldwide trend for earlier onset of puberty due to genetic factors, environmental factors, obesity, and endocrine disruptors that may lead to earlier releases of GnRH.7,8 Precocious puberty (PP) is characterized by pubertal changes occurring before the age of eight years in females and nine years in males.3,8 Central PP (CPP), as one of the etiologies of PP, is caused by early activation of the HPG axis either due to organic or idiopathic causes. Thus, secondary sexual characteristics follow the chronological sequence of normal puberty.7,8
Puberty is universally considered to be a stressful experience.7 Some research studies suggest that PP may result in psychosocial and behavioral problems in children including anxiety, depression, psychological stress, social withdrawal, and sleep problems, with a negative impact on their quality of life (QoL).1,9–12 However, others have mentioned normal behavior and psychosocial function in that cohort of children.13 The psychological consequences in girls with PP may be worse than those who mature at a normal age due to difficulties in coping with the psychological and physical changes that occur rapidly and prematurely outside the normal expected time.7 Associated body changes, in addition to hormonal changes, would cause the child to suffer from feelings of lower self-esteem and insecurity regarding their body image.14,15 Moreover, medical assessment including physical examination and treatment of children with CPP might cause fear and shame and have adverse effects on their psychological wellbeing.16
Treatment with GnRH analogues (GnRHa) influences the psychosocial functioning of children with CPP by blocking sex hormone production to delay pubertal development. Moreover, GnRHa can affect cognitive functioning through its receptors in brain areas unrelated to puberty.14 Studies have reported a varied prevalence of behavioral problems in children with CPP. Furthermore, some research studies have been trying to discuss psychological functioning in children with CPP treated with GnRHa. Some suggested that some of their psychological problems showed improvement after treatment. However, others showed that GnRHa did not affect psychological functioning.10,17 It remains unclear if psychological distress should be considered an expected consequence of PP supporting the decision to start treatment with GnRHa and whether the treatment could improve such stress. Thus, our study sought to estimate the impact of central PP on the behavior, psychosocial aspects, and QoL in these patients and to assess if there was a change after treatment as data on these aspects are limited in the literature.
Methods
Our case-control study included 30 CPP children following up in the Endocrinology Clinic of Alexandria University Children’s Hospital and compared them to 30 healthy age and sex-matched controls selected from outpatient clinics in the hospital. This sample size achieved 80% power to determine a difference of six in psychological aspects regarding anxiety scores between group 1 (41.2 ± 6.6) and group 2 (25.3 ± 4.1). This procedure used a two-sided independent samples t-test with a significance level of 0.05.18 Children with CPP were selected according to their established criteria including the onset of pubertal changes at an age less than eight years in girls and nine years in boys with bone age exceeding the chronological age, a peak luteinizing hormone (LH) level of ≥ 5 IU/L in the GnRH stimulation test.19 Excluded patients were those with other endocrinal or chronic diseases affecting cognitive or behavioral function, patients with known neurological or psychiatric disorders including epilepsy, childhood depression, learning difficulties, or autistic spectrum disorders, and children taking drugs that may affect the psychological or cognitive function. The study was approved by the Medical Ethics Committee of Alexandria Faculty of Medicine (no. 0106781). Informed consent was obtained from the parents of these children.
The CPP group was assessed with emphasis on history including age, sex, age at the onset of pubertal changes, presenting symptoms and signs, family history of PP, medical history of previous central nervous system disease, and drug history including exposure to sex steroids. Complete clinical examination was done with special emphasis on anthropometric measurements including weight, height, and body mass index, and their SD scores were assessed using the CDC 2000 growth reference.20 Physical changes of puberty were assessed at the onset and their sequence and staged by Tanner's sexual maturity rating.21 Hormonal assay included basal LH, basal follicular stimulating hormone (FSH), and sex steroids levels (estradiol and testosterone), and peak LH and FSH after GnRH stimulation test. Gonadotropin levels were estimated using immunochemiluminescent assay (ICMA) technique.22 In the GnRH stimulation test, blood samples for LH and FSH were collected at 0, 30, and 45 minutes after intramuscular injection of 100 mcg of GnRHa (triptorelin). Basal LH level > 0.3 IU/L and stimulated LH level ≥ 5 IU/L were used to detect the activation of the HPG axis.23 The pubertal cutoff for basal testosterone was > 25 ng/dL and for basal estradiol > 20 pg/mL.23
Imaging studies were done for all patients including bone age assessment by radiographs of the left wrist and hand, which was then assessed using the Greulich-Pyle atlas.24 A pelvic ultrasound (US) assessed the changes in uterus and ovaries and their dimensions. Brain magnetic resonance imaging was done to detect any possible central nervous system pathology. All CPP patients received subcutaneous Goserelin 3.6 mg prefilled syringe every 28 days.
The following instruments for neuropsychological assessment were used for both groups. First, the translated version of pediatric QoL inventoryTM version 4.0 (PedsQLTM) generic core scale was done to evaluate the disease and treatment effect on pediatric patients’ health-related QoL (HRQoL) during the preceding month.25,26 The report forms included a parent and guardians report form. The parent report form for toddlers (ages = 2–4) was composed of 21 items assessing four aspects: physical functioning (eight items), emotional functioning (five items), social functioning (five items), and school functioning (three items). The guardians report form for children (ages = 5–12) consisted of 23 items in the same four aspects and items except school functioning contained five items. Each item assessed the frequency of problems: 0 (never), 1 (almost never), 2 (sometimes), 3 (often), and 4 (almost). Then, items were linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Thus, higher scores mean better QoL. The total score was a summation of all items divided by the number of items. Second, the Child Behavior Checklist (CBCL) was used to assess child behavioral, and emotional problems.27 The parent report forms of the CBCL included: A) CBCL for ages 6–18 years rating childhood behavior on three categories (total, internalizing, and externalizing problems) and eight subcategories (withdrawn, somatic complaints, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior). The first three subcategories were added to the internalizing problems category and the last two to the externalizing problems category.27,28 B) CBCL for ages 1.5–5 years rating childhood behavior on three main categories (total, internalizing, and externalizing problems) and seven subcategories (withdrawn, somatic complaints, anxious/depressed, emotionally reactive, sleep problems, aggressive behavior, and attention problems). The first four subcategories were added up to the internalizing problems category and the last two to the externalizing problems.29 There was an Other Problems’, which listed specific problems that may be of clinical interest based on individual questions (ex., overeating, sleep problems). Finally, the total problems scale score consisted of all items.27 C) IQ testing was assessed by a translated Arabic version of the Stanford-Binet Intelligence Scale 4th edition for neurocognitive evaluation.30 The three tests will be repeated after one year for re-evaluation.
Data were analyzed using SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Qualitative data were described using numbers and percentages. The Shapiro-Wilk test was used to determine the normality of the distribution. Quantitative data were described using range (minimum and maximum), mean, SD, and median IQR. The significance of the obtained results was judged at the 5% level. The tests included chi-square test for categorical variables in comparison, Fisher’s exact or Monte Carlo correction for chi-square when > 20% of the cells have expected count < 5, Student’s t-test for normally distributed quantitative variables to compare between two studied groups, Mann–Whitney U-test for non-normally distributed quantitative variables to compare between two groups, and Friedman test in non-normally distributed quantitative variables to compare between more than two periods, and Post-hoc test (Dunn’s) for pairwise comparisons.
Results
The demographic data of 30 patients and 30 controls are summarized in Table 1. The most common presentation in the patient group was isolated thelarche in 15 (50.0%) patients. The male patient had increased penile length and pubic hair growth. The mean age of the study groups was 5.1 ± 2.3 years compared to 5.2 ± 2.2 years in the control group; 29 patients and 29 controls (96.7%) were females simulating epidemiologic characteristics of CPP. The mean height (SD) was higher in the case group. The age at onset of pubertal symptoms ranged from 1.0–9.5 years with a mean of 4.2 ± 2.5 years. The 9.5-year-old patient had menarche, so she was included in the study.
Table 1: Demographic characteristics of patients and controls.
|
Sex
|
|
Male
|
1 (3.3)
|
1 (3.3)
|
χ2 = 0.00
|
FE = 1.000
|
|
Female
|
29 (96.7)
|
29 (96.7)
|
|
|
|
Presenting symptoms
|
|
Isolated thelarche
|
15 (50.0)
|
|
|
|
|
Thelarche and adrenarche
|
8 (26.7)
|
|
|
|
|
Thelarche, adrenarche, menarche
|
6 (20.0)
|
|
|
|
|
Increased penile length, adrenarche
|
1 (3.3)
|
|
|
|
|
Age, years, mean ± SD
|
5.1 ± 2.3
|
5.2 ± 2.2
|
t = 0.143
|
0.887
|
|
Height, cm, mean ± SD
|
114.9 ± 19.7
|
108.2 ± 15.4
|
t = 1.459
|
0.150
|
|
Height, SD, mean ± SD
|
1.1 ± 1.2
|
-0.2 ± 0.8
|
t = 5.083*
|
< 0.001*
|
|
Weight, kg, mean ± SD
|
24.0 ± 12.7
|
19.4 ± 6.7
|
U = 348.50
|
0.133
|
|
BMI, kg/m2, mean ± SD
|
17.0 ± 3.3
|
16.0 ± 2.0
|
U = 344.00
|
0.117
|
t: Student’s t-test; χ2: chi-square test; FE: Fisher’s exact; U: Mann-Whitney test.
*Statistically significant at p ≤ 0.05.
Table 2 shows Tanner staging of pubertal changes during presentation in CPP patients. Stage 3 of breast development (B3) was the most common presenting stage in 21 (72.4%) cases. Bone age was advanced in 21 (70.0%) patients.
Table 2: Distribution of the studied patients according to Tanner staging of pubertal changes at presentation and radiological investigations.
|
Tanner staging
|
|
Breast enlargement (n = 29)a
|
|
B1
|
0 (0.0)
|
|
B2
|
1 (3.4)
|
|
B3
|
21 (72.4)
|
|
B4
|
7 (24.1)
|
|
Pubic hair (n = 30)
|
|
T1
|
16 (53.3)
|
|
T2
|
7 (23.3)
|
|
T3
|
6 (20.0)
|
|
T4
|
1 (3.3)
|
|
Axillary hair (n = 30)
|
|
Absent
|
23 (76.7)
|
|
Present
|
7 (23.3)
|
|
Radiological investigations
|
|
Bone age at diagnosis, years
|
|
Not advanced
|
9 (30.0)
|
|
Advanced
|
21 (70.0)
|
|
(BA-CA) ≤ 2 years
|
8 (38.1)
|
|
(BA-CA) > 2 year
|
13 (61.9)
|
|
Min–max
|
1.0–13.0
|
|
Mean ± SD
|
5.9 ± 3.1
|
|
Median (IQR)
|
6.5 (3.0–9.0)
|
|
US genitals
|
|
|
Prepubertal
|
14 (46.7)
|
a: Number of females; BA-CA: ; US: ultrasound.
While assessing the hormonal profile of CPP cases, Figures 1 and 2 showed a statistically significant increase in the peak LH and FSH (at 30 minutes and 45 minutes after stimulation with GnRH analogues) compared to their basal levels in patients with CPP. Five cases did not do a GnRH stimulation test due to their elevated basal LH level and other clinical criteria. There was a statistically significant rise in the levels of LH and FSH after GnRH stimulation. Among 25 cases who did the GnRH stimulation test, 24 (96.0%) showed a pubertal response while one case (4.0%) showed a prepubertal response. Still, other clinical criteria with pubertal changes of the uterus and ovaries on the pelvic US matched with the diagnosis of CPP.
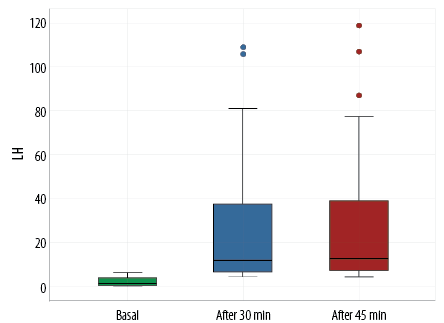 Figure 1: Comparison between basal and peak luteinizing hormone (LH) levels after gonadotropin-releasing hormone stimulation in the central precocious puberty group.
Figure 1: Comparison between basal and peak luteinizing hormone (LH) levels after gonadotropin-releasing hormone stimulation in the central precocious puberty group.
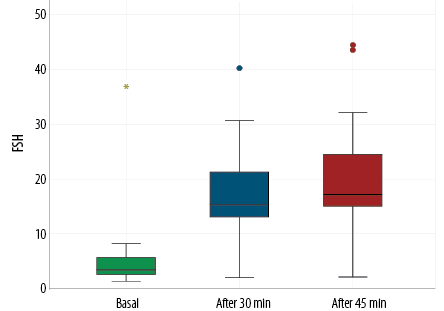 Figure 2: Comparison between basal and peak follicular stimulating hormone (FSH) levels after gonadotropin-releasing hormone stimulation in the central precocious puberty group.
Figure 2: Comparison between basal and peak follicular stimulating hormone (FSH) levels after gonadotropin-releasing hormone stimulation in the central precocious puberty group.
Table 3 shows the PedsQLTM 4.0 GCS scores of the two groups. Children with CPP scored less than controls in all HRQoL domains except school functioning.
Table 3 compares CBCL T-scores between cases and control groups. The CBCL scores including internalizing problems, externalizing problems, and their subscales were not significantly higher in cases than controls. Furthermore, there was no statistically significant difference between them in total behavior problem scores, other behavior problem scores, social problems, attention problems, thought problems, and sleep problems.
Table 3: Comparison of PedsQL™ 4.0 GCS scores and CBCL T-scores between the cases and control groups.
|
Pediatric quality of life
|
|
|
|
|
|
Physical health summary score
|
65.7 ± 10.8
|
77.5 ± 10.6
|
t = 4.250*
|
< 0.001*
|
|
Emotional functioning
|
60.6 ± 14.2
|
74.0 ± 13.4
|
t = 3.723*
|
< 0.001*
|
|
Social functioning
|
66.1 ± 10.0
|
77.6 ± 12.0
|
U = 211.50
|
< 0.001*
|
|
School functioning
|
63.9 ± 14.5
|
72.0 ± 15.6
|
t = 1.756
|
0.087
|
|
Psychosocial summary score
|
63.4 ± 8.3
|
75.5 ± 9.9
|
t = 1.756
|
< 0.001*
|
|
Total summary score
|
64.2 ± 7.6
|
76.2 ± 9.0
|
t = 5.535
|
< 0.001*
|
|
CBCL
|
|
|
|
|
|
Withdrawn
|
57.6 ± 9.8
|
54.3 ± 6.9
|
U = 337.50
|
0.075
|
|
Somatic complaint
|
55.7 ± 10.1
|
53.8 ± 7.5
|
U = 444.50
|
0.926
|
|
Anxious/depressed
|
57.8 ± 9.6
|
56.0 ± 8.8
|
U = 393.50
|
0.375
|
|
Emotionally reactive
|
56.9 ± 10.2
|
52.8 ± 5.95
|
U = 101.50
|
0.331
|
|
Internalizing problems
|
51.0 ± 17.7
|
46.4 ± 17.0
|
U = 380.00
|
0.297
|
|
Delinquent behavior
|
53.3 ± 8.1
|
53.4 ± 6.0
|
U = 94.00
|
0.652
|
|
Aggressive behavior
|
55.7 ± 7.5
|
53.7 ± 7.7
|
U = 350.50
|
0.107
|
|
Externalizing problems
|
46.8 ± 14.8
|
42.7 ± 14.1
|
U = 384.50
|
0.327
|
|
Social problems
|
58.5 ± 9.2
|
56.7 ± 7.6
|
U = 84.50
|
0.555
|
|
Thought problems
|
57.2 ± 8.9
|
52.8 ± 6.1
|
U = 73.50
|
0.274
|
|
Attention problems
|
56.7 ± 7.7
|
54.4 ± 6.8
|
U = 352.00
|
0.118
|
|
Sleep problems
|
55.8 ± 9.0
|
53.6 ± 5.4
|
U = 109.50
|
0.502
|
|
Other problems
|
6.2 ± 7.2
|
5.0 ± 5.3
|
U = 433.50
|
0.805
|
t: Student t-test; U: Mann-Whitney U test; CBCL: child behavior checklist.
*Statistically significant at p ≤ 0.05.
According to Stanford Binet intelligence scale score, the mean estimated total IQR score was 89.3 ± 8.8 (range = 76.0–116.0) for cases and 90.3 ± 11.5 (range = 72.0–116.0) for the control group. The difference between them was not statistically significant in all subscales.
Table 4 shows that children with CPP had improved scores after treatment with GnRHa for one year in all HRQoL domains especially physical and emotional functioning compared to their scores pre-treatment. The mean PedsQLTM T-score was (64.2 ± 7.6) at the time of diagnosis versus (70.1 ± 7.7) at follow-up post-treatment and this difference was statistically significant (p = 0.002).
Table 4: Comparison of pediatric quality of life scores before and after treatment in patients’ group.
|
Physical health summary score
|
65.7 ± 10.8
|
72.5 ± 9.6
|
t = 3.367*
|
0.002*
|
|
Emotional functioning
|
60.6 ± 14.2
|
67.8 ± 11.3
|
t = 3.288*
|
0.003*
|
|
Social functioning
|
66.1 ± 10.0
|
70.1 ± 10.8
|
Z = 2.079*
|
0.038*
|
|
School functioning
|
63.9 ± 14.5
|
67.7 ± 10.4
|
t = 2.242*
|
0.033*
|
|
Psychosocial summary score
|
63.4 ± 8.3
|
68.8 ± 8.3
|
t = 3.179
|
0.004*
|
t: paired t-test; Z: Wilcoxon signed ranks test.
Table 5 shows a comparison of CBCL T-scores initially and one year post-treatment with GnRHa. In all CPP patients, all clinical manifestations were well suppressed by treatment including regression of Tanner staging and menstruation was stopped if present. Total behavioral problems score and other problems score showed significant improvement after treatment. The score of internalizing problems and its subscales improved after treatment and showed statistically significant differences except in the emotionally reactive subscale. Scores of externalizing problems and their aggressive behavior subscale significantly enhanced after treatment. After treatment, 27 (90.0%) cases got total behavior problems classification at a normal range compared to 23 (76.7%) cases before treatment. Three out of five cases improved in total behavior problems score after treatment from being at a clinical range to a normal range. One out of two cases got an improved total behavior problems score from being at a borderline range to a normal range.
Table 5: Comparison of CBCL T-scores before and after treatment in cases group.
|
Withdrawn
|
57.6 ± 9.8
|
53.0 ± 4.5
|
3.062*
|
0.002*
|
|
Somatic complaint
|
55.7 ± 10.1
|
53.0 ± 5.9
|
2.002*
|
0.045*
|
|
Anxious/depressed
|
57.8 ± 9.6
|
54.2 ± 5.9
|
2.982*
|
0.003*
|
|
Emotionally reactive
|
56.9 ± 10.2
|
53.7 ± 7.1
|
1.461
|
0.144
|
|
Internalizing problems
|
51.0 ± 17.7
|
46.0 ± 14.2
|
3.182*
|
0.001*
|
|
Delinquent behavior
|
53.3 ± 8.1
|
53.8 ± 8.1
|
0.365
|
0.715
|
|
Aggressive behavior
|
55.7 ± 7.5
|
53.7 ± 6.1
|
2.137*
|
0.033*
|
|
Externalizing problems
|
|
Min–max
|
28.0–68.0
|
28.0 – 68.0
|
2.319*
|
0.020*
|
|
Mean ± SD
|
46.8 ± 14.8
|
44.23 ± 13.2
|
|
|
|
Median (IQR)
|
48.5 (28.0–59.0)
|
43.0 (28.0–56.0)
|
|
|
|
Social problems
|
58.5 ± 9.2
|
55.8 ± 5.6
|
1.757
|
0.079
|
|
Thought problems
|
57.2 ± 8.9
|
53.4 ± 5.3
|
1.472
|
0.141
|
|
Attention problems
|
56.7 ± 7.7
|
54.7 ± 6.3
|
1.780
|
0.075
|
|
Sleep problems
|
55.8 ± 9.0
|
54.3 ± 7.4
|
0.730
|
0.465
|
Z: Wilcoxon signed ranks test. *Statistically significant at p ≤ 0.05.
Regarding internalizing problems, 27 cases got internalizing problems classification at a normal range compared to 20 cases before treatment. Five out of seven cases improved after treatment from borderline range to normal range and two out of three cases improved from clinical to normal range. As for the classification of externalizing problems, two out of three cases improved from borderline to normal range. Thus, increasing the number in the normal range from 27 to 29 cases.
The Stanford Binet Intelligence Scale scores were used for cognitive function assessment. The mean estimated total IQ score was 89.3 ± 8.8 (range = 76.0–116.0) for cases and 90.3 ± 11.5 (range = 72.0–116.0) for the control group. The difference was not statistically significant. Also, all subscales showed no statistically significant difference between cases and controls.
Discussion
Puberty may have a great physical and psychosocial impact on children, especially when maturation occurs earlier than expected.31 It is frequently asked if GnRHa treatment is important to alleviate psychological distress associated with PP. However, results from studies performed to date have varied. Some studies found that early puberty results in psychological distress, and social and behavioral problems with a negative impact on the QoL. However, others reported normal behavior and psychosocial function in children with sexual precocity.7,10,17
This study investigated the impact of CPP on the psychosocial, behavioral, cognitive aspects, and QoL in CPP children. Moreover, the study aimed to determine the changes in these psychosocial aspects after treatment with GnRHa. This study was conducted on 30 children with CPP and 30 healthy children of matched age and sex. According to demographic data, 29 (96.7%) patients were females with a female-to-male ratio of 29:1. The mean age of children with CPP was 5.1 ± 2.3 years. All children were diagnosed before eight years except one girl diagnosed at 9.5 years with menarche after the appearance of other sexual characteristics earlier. There were variable presentations in this cohort. Breast enlargement alone was the most common presentation in 13 (43.3%) cases. The GnRH test was not done in five patients diagnosed with elevated basal LH, clinical criteria, and advanced bone age. Among cases who did the GnRH stimulation, 24 (96.0%) patients showed pubertal response while one (4.0%) patient showed prepubertal response with positive height SD (+0.6), T2 breast, no pubic or axillary hair and normal bone age, but pelvic US showed pubertal changes of the uterus and ovaries. This may be explained by low LH response to GnRH in early puberty at breast stage 2 to early 3.1
Children with CPP demonstrated poorer QoL. They performed worse than controls in all domains except school functioning. Similarly, there have been several studies reported that children with CPP had a poorer QoL than controls.32–34 Klein et al,32 carried out a cross-sectional study on 142 parents of CPP children in the USA and found that children with CPP got significantly poorer scores than the control group in HRQoL domains. Similarly, a case-control study carried out in China revealed that children with CPP got significantly lower scores than the control group in HRQoL domains except for physical functioning.34 Another longitudinal study by Mensah et al,33 performed a psychosocial assessment in Australian children with CPP using PedsQLTM. They found that children with CPP had lower psychosocial health scores than children with normal puberty and reported lower scores for all other PedsQLTM sub-scores. In contrast, a study35 carried in Antalya on 71 girls with CPP and 50 control girls found no significant difference in mean PedsQLTM scores between cases and controls.35 This difference may be due to the cultural difference of the instruments and the start of treatment. However, few studies were conducted to assess whether the adverse effects of HRQoL described in children with CPP improvedwith treatment. Thus, this study showed that, children with CPP got significantly higher scores after treatment in all HRQoL domains, especially physical and emotional functioning domains. On review of previous studies, Klein et al,32 conducted an online survey for CPP children. Eighty-six children out of 142 patients received treatment. They reported that PedsQLTM scores did not show significant differences between children who were never treated and those who received treatment either at the time of the study or in the past. The difference in results may be attributed to the fact that the current study was a prospective study and the same patient was evaluated before and after treatment which gave more accurate observations.
In the current study, children with CPP did not show significantly more behavioral problems than the control group. Scores of internalizing problems and their subscales, scores of externalizing problems and their subscales, and total behavioral problems scores were higher in children with CPP than controls with no statistically significant difference. Williams et al,9 reviewed the instruments used for psychosocial assessments of children with CPP. They mentioned that several observational studies used CBCL for psychological assessment. Among the studies mentioned, some CBCL subscales showed significant problems in children with CPP compared to healthy controls, but others found no significant differences. Similarly, a study carried out by Mul et al,36 on adopted children with early puberty found that they did not have increased levels of behavioral or emotional problems by CBCL. Two other studies conducted on girls with CPP found that they have no statistically significant behavioral problems compared to the control group.14, 37
In contrast, older studies showed different levels of behavioral problems in children with CPP. Sonis et al,38 Xhrouet-Heinrichs et al,10 and Kim et al,1 reported that girls with CPP had behavioral problems in comparison to their controls. The discrepancy between the current results and the above-mentioned latter three studies may be due to differences in the age of children, duration of treatment, and time of assessment. This observed decrease in behavioral problems in the recent studies (including the current study) could result from less stigmatization of this condition and the current rationale towards early diagnosis, treatment, and ongoing monitoring.14
There are few studies investigating the impact of GnRHa treatment on the psychological features of children with PP. In this study, we found that the total behavior problems score, internalizing problems and theirs subscales, except for the emotionally reactive subscale, externalizing problems and their aggressive behavior subscale, and other problems scores improved significantly after treatment. Scores of delinquent behavior, attention problems, social problems, thought problems, and sleep problems showed non-significant improvement after treatment. In terms of clinically important T-score, 60.0% of patients improved in the total behavior problems score after treatment from being at a clinical range to a normal range. Similarly, Kim et al,39 followed 54 girls with CPP during treatment over 24 months to detect clinical, laboratory, and radiological responses to treatment and changes in psychological aspects after treatment. They found that total behavior problems scores and other problems scores were significantly lower after treatment. However, anxiety/depression, somatic complaints, attention problems, social problems, and thought problems scores did not change significantly after treatment. This improvement in total behavior problem scores may be related to the effect of treatment on improving physical changes associated with PP, regular endocrinology clinic visits, and counseling.39
In contrast, Wojniusz et al,14 conducted a study on 15 treated girls with CPP and 15 control girls and found that treated girls with CPP did not differ in their psychological or cognitive functioning from controls.
With regards the effect of CPP on cognitive function, there was no statistically significant difference between the total IQ score and its subscores between cases and controls. Similarly, Wojniusz et al,14 and Mul et al,36 assessed the cognitive function in girls with CPP and controls using Wechsler Intelligence Scale for Children-III and found no significant difference in the mean IQ score between the two groups.
Moreover, this study demonstrated that children with advanced pubertal development (B4, pubic hair T2-T4, and presence of menses) showed significantly lower social functioning, psychosocial health, and total health scores. These results are consistent with the possible effect of physical pubertal changes in early-maturing girls on their social functioning.7
One of the limitations of our study was the small sample size. Moreover, we relied upon the CBCL and PedQLTM completed by the parents, not by the children themselves as there were children diagnosed with CPP as young as two years of age.
Conclusion
QoL is significantly affected in children with CPP. However, children with CPP did not have significantly more behavioral problems or cognitive dysfunction than their healthy peers. After treatment with GnRHa, all pediatric QoL domains and CBCL T-scores showed significant improvement. Thus, this study raised awareness of the importance of psychosocial assessment and early treatment of children with CPP to improve their QoL and behavior.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
The study group is very grateful to the children and parents who supported this study.
references
- 1. Kim EY, Lee MI. Psychosocial aspects in girls with idiopathic precocious puberty. Psychiatry Investig 2012 Mar;9(1):25-28.
- 2. Vigil P, Orellana RF, Cortés ME, Molina CT, Switzer BE, Klaus H. Endocrine modulation of the adolescent brain: a review. J Pediatr Adolesc Gynecol 2011 Dec;24(6):330-337.
- 3. Chirico V, Lacquaniti A, Salpietro V, Buemi M, Salpietro C, Arrigo T. Central precocious puberty: from physiopathological mechanisms to treatment. J Biol Regul Homeost Agents 2014;28(3):367-375.
- 4. Soriano-Guillén L, Argente J. Central precocious puberty, functional and tumor-related. Best Pract Res Clin Endocrinol Metab 2019 Jun;33(3):101262.
- 5. Uenoyama Y, Tsukamura H, Maeda K. KNDy neuron as a gatekeeper of puberty onset. J Obstet Gynaecol Res 2014 Jun;40(6):1518-1526.
- 6. Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol 2017 Jan;44:122-137.
- 7. Mercader-Yus E, Neipp-López MC, Gómez-Méndez P, Vargas-Torcal F, Gelves-Ospina M, Puerta-Morales L, et al. Anxiety, self-esteem and body image in girls with precocious puberty. Rev Colomb Psiquiatr (Engl Ed) 2018;47(4):229-236.
- 8. Garibaldi LR, Chemaitilly W. Disorders of pubertal development. In: Kliegman R, Stanton B, Geme J, Schor N, Behrman R, Nelson M, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016. p. 2656-2662.
- 9. Williams VS, Soliman AM, Barrett AM, Klein KO. Review and evaluation of patient-centered psychosocial assessments for children with central precocious puberty or early puberty. J Pediatr Endocrinol Metab 2018 Apr;31(5):485-495.
- 10. Xhrouet-Heinrichs D, Lagrou K, Heinrichs C, Craen M, Dooms L, Malvaux P, et al. Longitudinal study of behavioral and affective patterns in girls with central precocious puberty during long-acting triptorelin therapy. Acta Paediatr 1997 Aug;86(8):808-815.
- 11. Mrug S, Elliott MN, Davies S, Tortolero SR, Cuccaro P, Schuster MA. Early puberty, negative peer influence, and problem behaviors in adolescent girls. Pediatrics 2014 Jan;133(1):7-14.
- 12. Turan AP, Akca SO. The quality of life of children with precocious puberty and healthy children in Turkey. Am J Health Behav 2021 Jan;45(1):62-70.
- 13. Tremblay L, Frigon JY. Precocious puberty in adolescent girls: a biomarker of later psychosocial adjustment problems. Child Psychiatry Hum Dev 2005;36(1):73-94.
- 14. Wojniusz S, Callens N, Sütterlin S, Andersson S, De Schepper J, Gies I, et al. Cognitive, emotional, and psychosocial functioning of girls treated with pharmacological puberty blockage for idiopathic central precocious puberty. Front Psychol 2016 Jul;7:1053.
- 15. Waylen A, Wolke D. Sex ‘n’ drugs ‘n’ rock ‘n’ roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol 2004 Nov;151(Suppl 3):U151-U159.
- 16. Baumann DA, Landolt MA, Wetterwald R, Dubuis JM, Sizonenko PC, Werder EA. Psychological evaluation of young women after medical treatment for central precocious puberty. Horm Res 2001;56(1-2):45-50.
- 17. Schoelwer MJ, Donahue KL, Didrick P, Eugster EA. One-year follow-up of girls with precocious puberty and their mothers: do psychological assessments change over time or with treatment? Horm Res Paediatr 2017;88(5):347-353.
- 18. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017.
- 19. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al; ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009 Apr;123(4):e752-e762.
- 20. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics 2002 Jan;109(1):45-60.
- 21. Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med 1968;19:283-300.
- 22. Chen W, Jie WU, Chen ZO, Jie XU, Huang-Xian JU. Chemiluminescent immunoassay and its applications. Chin J Anal Chem 2012;40(1):3-10.
- 23. Ab Rahim SN, Omar J, Tuan Ismail TS. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: a mini review. Ann Pediatr Endocrinol Metab 2020 Sep;25(3):152-155.
- 24. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: CA: Stanford University Press and London; 1959.
- 25. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001 Aug;39(8):800-812.
- 26. Arabiat D, Elliott B, Draper P, Al Jabery M. Cross-cultural validation of the pediatric quality of life inventory™ 4.0 (PedsQL™) generic core scale into Arabic language. Scand J Caring Sci 2011 Dec;25(4):828-833.
- 27. Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment. Burlington n, VT: University of Vermont. Research Center for Children, Youth, & Families; 2001. p. 1617.
- 28. Ibrahim N, Wahdan I, Abou-Nazel MW, Sallam SA. Psychometric properties and diagnostic accuracy of the Arabic version of the strngths and difficulties questionnaire and the child behavior checklist, in epide-miology. Master Thesis: High Institute of Public Health, Alexandria University; 2016.
- 29. Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont. Research Center for Children, Youth, & Families; 2000.
- 30. Melika L. Stanford Binet. Arabic version. 4th ed. Cairo, Egypt: Dar El-Nahda Al-Arabia; 1998.
- 31. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol 2016 Mar;4(3):254-264.
- 32. Klein KO, Soliman AM, Grubb E, Nisbet P. A survey of care pathway and health-related quality of life impact for children with central precocious puberty. Curr Med Res Opin 2020 Mar;36(3):411-418.
- 33. Mensah FK, Bayer JK, Wake M, Carlin JB, Allen NB, Patton GC. Early puberty and childhood social and behavioral adjustment. J Adolesc Health 2013 Jul;53(1):118-124.
- 34. Yang H, Luo S, Liang X, Lin Q, Cheng T, Zeng L, et al. The association between family impact and health-related quality of life of children with idiopathic central precocious puberty in Chongqing, China. Health Qual Life Outcomes 2021 Jun;19(1):171.
- 35. Çoban OG, Bedel A, Önder A, Adanır AS, Tuhan H, Parlak M. Psychiatric disorders, peer-victimization, and quality of life in girls with central precocious puberty. J Psychosom Res 2021 Apr;143:110401.
- 36. Mul D, Versluis-den Bieman HJ, Slijper FM, Oostdijk W, Waelkens JJ, Drop SL. Psychological assessments before and after treatment of early puberty in adopted children. Acta Paediatr 2001 Sep;90(9):965-971.
- 37. Schoelwer MJ, Donahue KL, Bryk K, Didrick P, Berenbaum SA, Eugster EA. Psychological assessment of mothers and their daughters at the time of diagnosis of precocious puberty. Int J Pediatr Endocrinol 2015;2015(1):5.
- 38. Sonis WA, Comite F, Blue J, Pescovitz OH, Rahn CW, Hench KD, et al. Behavior problems and social competence in girls with true precocious puberty. J Pediatr 1985 Jan;106(1):156-160.
- 39. Kim YJ, Lee HS, Lee YJ, Lim JS, Kim SY, Kim EY, et al. Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 2013 Dec;18(4):173-178.